Abstract
Introduction
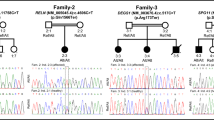
Lafora disease (LD) is a severe form of progressive myoclonus epilepsy characterized by generalized seizures, myoclonus, intellectual decline, ataxia, spasticity, dysarthria, visual loss, and in later stages, psychosis and dementia. To date, mutations in the EPM2A and EPM2B/NHLRC1 genes have been identified as the common causes of LD. However, a mutation in PRDM8 has been reported only once in a Pakistani family affected with early-onset Lafora disease. In the present study, we report the second family with a PRDM8 mutation.
Methods
Two affected individuals of an Iranian family initially diagnosed as complicated hereditary spastic paraplegia (HSP) underwent careful neurologic examination. Homozygosity mapping and whole-exome sequencing were performed. Based on the results of genetic analysis to detection of Lafora bodies, a skin biopsy was done.
Results
The clinical features of the patients were described. Linkage to chromosome 4 and a mutation in the PRDM8 gene were identified, suggesting the patients may be affected with early-onset LD. However, like the Pakistani family, the search for Lafora bodies in their skin biopsies was negative. Their electroencephalograms showed generalized epileptiform discharges in the absence of clinical seizures.
Conclusions
The current study increases the number of PRDM8-related cases and expands the phenotypic spectrum of mutations in the PRDM8 gene. Both reported PRDM8-related families presented intra and inter-familial heterogeneity and they have originated from the Middle East. Thus, it seems the PRDM8 mutations should be considered not only in LD but also in other neurodegenerative disorders such as a complicated HSP-like phenotype, especially in this region.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Jansen AC, Andermann E (2019) Progressive myoclonus epilepsy, Lafora type, GeneReviews®[Internet]. University of Washington, Seattle
Al Mufargi Y, Qureshi A, Al Asmi A (2020) Lafora disease: report of a rare entity. Cureus 12(1):e6793
Minassian BA (2001) Lafora’s disease: towards a clinical, pathologic, and molecular synthesis. Pediatr Neurol 25(1):21–29
Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-García O, Fernandez-Sanchez E, Medraño-Fernández I, Domínguez J (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10(11):1407–1413
Nitschke F, Ahonen SJ, Nitschke S, Mitra S, Minassian BA (2018) Lafora disease—from pathogenesis to treatment strategies. Nat Rev Neurol 14(10):606–617
Nitschke F, Ahonen SJ, Nitschke S, Mitra S, Minassian BAJNRN (2018) Lafora disease—from pathogenesis to treatment strategies. Nat Rev Neurol 14(10):606–617
Delgado-Escueta A, Ganesh S, Yamakawa K (2001) Advances in the genetics of progressive myoclonus epilepsy. Am J Med Genet 106(2):129–138
Brewer MK, Putaux J-L, Rondon A, Uittenbogaard A, Sullivan MA, Gentry MS (2020) Polyglucosan body structure in Lafora disease. Carbohydr Polym 240:116260
Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS (2012) Glycogen and its metabolism: some new developments and old themes. Biochem J 441(3):763–787
Delgado-Escueta AV (2007) Advances in lafora progressive myoclonus epilepsy. Curr Neurol Neurosci Rep 7(5):428–433
Tagliabracci VS, Girard JM, Segvich D, Meyer C, Turnbull J, Zhao X, Minassian BA, DePaoli-Roach AA, Roach PJ (2008) Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J Biol Chem 283(49):33816–33825
Turnbull J, Girard J-M, Lohi H, Chan EM, Wang P, Tiberia E, Omer S, Ahmed M, Bennett C, Chakrabarty A (2012) Early-onset Lafora body disease. Brain 135(9):2684–2698
Chan EM, Young EJ, Ianzano L, Munteanu I, Zhao X, Christopoulos CC, Avanzini G, Elia M, Ackerley CA, Jovic NJJNG (2003) Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet 35(2):125–127
Turnbull J, DePaoli-Roach AA, Zhao X, Cortez MA, Pencea N, Tiberia E, Piliguian M, Roach PJ, Wang P, Ackerley CA (2011) PTG depletion removes Lafora bodies and rescues the fatal epilepsy of Lafora disease. PLoS Genet 7(4):e1002037
Serratosa JM, Gómez-Garre P, Gallardo ME, Anta B, De Bernabé DB-V, Lindhout D, Augustijn PB, Tassinari CA, Michelucci R, Malafosse AJHMG (1999) A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2). Hum Mol Genet 8(2):345–352
Chan E, Omer S, Ahmed M, Bridges L, Bennett C, Scherer S, Minassian BJN (2004) Progressive myoclonus epilepsy with polyglucosans (Lafora disease): evidence for a third locus. Neurology 63(3):565–567
Ganesh S, Delgado-Escueta AV, Sakamoto T, Avila MR, Machado-Salas J, Hoshii Y, Akagi T, Gomi H, Suzuki T, Amano K (2002) Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum Mol Genet 11(11):1251–1262
Nitschke F, Sullivan MA, Wang P, Zhao X, Chown EE, Perri AM, Israelian L, Juana-López L, Bovolenta P, Rodríguez de Córdoba S (2017) Abnormal glycogen chain length pattern, not hyperphosphorylation, is critical in Lafora disease. EMBO Mol Med 9(7):906–917
Singh S, Satishchandra P, Shankar SK, Ganesh S (2008) Lafora disease in the Indian population: EPM2A and NHLRC1 gene mutations and their impact on subcellular localization of laforin and malin. Hum Mutat 29(6):E1–E12
van de Peppel J, Holstege FC (2005) Multifunctional genes. Mol Syst Biol 1(1):2005.0003
Inoue M, Kuroda T, Honda A, Komabayashi-Suzuki M, Komai T, Shinkai Y, Mizutani K-I (2014) Prdm8 regulates the morphological transition at multipolar phase during neocortical development. PLoS One 9(1):e86356
Jung CC, Atan D, Ng D, Ploder L, Ross SE, Klein M, Birch DG, Diez E, McInnes RR (2015) Transcription factor PRDM8 is required for rod bipolar and type 2 OFF-cone bipolar cell survival and amacrine subtype identity. Proc Natl Acad Sci 112(23):E3010–E3019
Orouji E, Peitsch WK, Orouji A, Houben R, Utikal J (2020) Unique role of histone methyltransferase PRDM8 in the tumorigenesis of virus-negative Merkel cell carcinoma. Cancers 12(4):1057
Park J, Kwon SO, Kim S-H, Kim SJ, Koh EJ, Won S, Kim WJ, Hwang SY (2020) Methylation quantitative trait loci analysis in Korean exposome study. Mol Cell Toxicol 16:175–183
Neidhart M, Pajak A, Laskari K, Riksen NP, Joosten LA, Netea MG, Lutgens E, Stroes ES, Ciurea A, Distler O (2019) Oligomeric S100A4 is associated with monocyte innate immune memory and bypass of tolerance to subsequent stimulation with lipopolysaccharides. Front Immunol 10:791
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector EJGIM (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–423
Ferlazzo E, Canafoglia L, Michelucci R, Gambardella A, Gennaro E, Pasini E, Riguzzi P, Plasmati R, Volpi L, Labate A (2014) Mild L afora disease: clinical, neurophysiologic, and genetic findings. Epilepsia 55(12):e129–e133
Ross SE, McCord AE, Jung C, Atan D, Mok SI, Hemberg M, Kim T-K, Salogiannis J, Hu L, Cohen SJN (2012) Bhlhb5 and Prdm8 form a repressor complex involved in neuronal circuit assembly. Neuron 73(2):292–303
Funding
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All participants, after being informed of the nature of the research, consented to participate. This research was performed in accordance with the Declaration of Helsinki and with approval of the ethics board of the University of Social Welfare and Rehabilitation Sciences.
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file6 (AVI 13667 KB)
Supplementary file7 (AVI 6323 KB)
Rights and permissions
About this article
Cite this article
Davarzani, A., Shahrokhi, A., Hashemi, S.S. et al. The second family affected with a PRDM8-related disease. Neurol Sci 43, 3847–3855 (2022). https://doi.org/10.1007/s10072-021-05815-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05815-w