Abstract
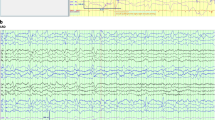
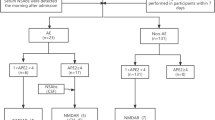
Antibody-mediated encephalitides constitute a group of inflammatory brain diseases characterized by prominent neuropsychiatric symptoms and are associated with antibodies against neuronal cell-surface proteins, ion channels, or receptors. The diagnosis and management of autoimmune encephalitis include evaluation of the clinical presentation, brain imaging, cerebrospinal fluid (CSF) findings, antibody detection, and electroencephalography (EEG) findings. This is a retrospective study of adults 18 years or older with autoimmune encephalitis due to antibodies against membrane surface antigens as well as anti-glutamic acid decarboxylase (anti-GAD) antibodies. The electronic medical record was reviewed for demographic data, clinical data, laboratory results, EEG, and imaging findings. Antibody screening was requested for 341 patients between May 2014 and December 2019. Antibody screening was positive in 37 patients presenting with seizures and/or encephalopathy. Of these, 10 patients tested positive for antibodies against neuronal surface antigens or anti-GAD antibodies―2 patients had anti-GAD antibody encephalitis, 5 had anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis, and 3 had anti-leucine-rich glioma-inactivated 1 (anti-LGI1) encephalitis. Demographics, clinical presentation, EEG, imaging, and CSF findings are reported. Autoimmune encephalitides are a diverse group of disorders with a few common clinical features and MRI findings. MRI, EEG, and CSF findings can be normal or show nonspecific findings in autoimmune encephalitis. Therefore, early diagnosis of these disorders requires a high level of suspicion to avoid delaying the diagnosis. Carefully looking for diagnostic clinical features (e.g., faciobrachial dystonic seizures in anti-LGI1 encephalitis), significant findings in MRI (e.g., limbic encephalitis), and some EEG patterns (e.g., extreme delta brush and generalized rhythmic delta activity in anti-NMDAR encephalitis) may help in early diagnosis.





Similar content being viewed by others
References
Dalmau J (2016) NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: the 2016 Cotzias Lecture. Neurology 87(23):2471–2482. https://doi.org/10.1212/WNL.0000000000003414
Dalmau J, Graus F (2018) Antibody-mediated encephalitis. N Engl J Med 378(9):840–851. https://doi.org/10.1056/NEJMra1708712
Dubey D, Pittock SJ, Kelly CR et al (2018) Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 83(1):166–177. https://doi.org/10.1002/ana.25131
Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS, UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group (2010) Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 10(12):835–44. https://doi.org/10.1016/S1473-3099(10)70222-X. Erratum in: Lancet Infect Dis. 2011 Feb;11(2):79
Vogrig A, Joubert B, André-Obadia N, Gigli GL, Rheims S, Honnorat J (2019) Seizure specificities in patients with antibody-mediated autoimmune encephalitis. Epilepsia 60(8):1508–1525. https://doi.org/10.1111/epi.16282
Gu Y, Zhong M, He L et al (2019) Epidemiology of antibody-positive autoimmune encephalitis in Southwest China: a multicenter study. Front Immunol 10:2611. https://doi.org/10.3389/fimmu.2019.02611
Titulaer MJ, McCracken L, Gabilondo I et al (2013) Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12(2):157–165. https://doi.org/10.1016/S1474-4422(12)70310-1
van Sonderen A, Petit-Pedrol M, Dalmau J, Titulaer MJ (2017) The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat Rev Neurol 13(5):290–301. https://doi.org/10.1038/nrneurol.2017.43
Fredriksen JR, Carr CM, Koeller KK, Verdoorn JT, Gadoth A, Pittock SJ, Kotsenas AL (2018) MRI findings in glutamic acid decarboxylase associated autoimmune epilepsy. Neuroradiology 60(3):239–245. https://doi.org/10.1007/s00234-018-1976-6
Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA (2012) The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis 54(7):899–904. https://doi.org/10.1093/cid/cir1038
Saraya AW, Worachotsueptrakun K, Vutipongsatorn K, Sonpee C, Hemachudha T (2019) Differences and diversity of autoimmune encephalitis in 77 cases from a single tertiary care center. BMC Neurol 19(1):273. https://doi.org/10.1186/s12883-019-1501-5
Gadoth A, Pittock SJ, Dubey D, McKeon A, Britton JW, Schmeling JE, Smith A, Kotsenas AL, Watson RE, Lachance DH, Flanagan EP, Lennon VA, Klein CJ (2017) Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol 82(1):79–92. https://doi.org/10.1002/ana.24979
Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C (2015) Imaging of autoimmune encephalitis—relevance for clinical practice and hippocampal function. Neuroscience 309:68–83. https://doi.org/10.1016/j.neuroscience.2015.05.037
Kallini JR, Hamed N, Khachemoune A (2015) Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol 54(2):130–140. https://doi.org/10.1111/ijd.12553
Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG (2010) Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 67(4):470–478. https://doi.org/10.1002/ana.21917
Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D (2012) Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 79(11):1094–1100. https://doi.org/10.1212/WNL.0b013e3182698cd8
Bacchi S, Franke K, Wewegama D, Needham E, Patel S, Menon D (2018) Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: a systematic review. J Clin Neurosci 52:54–59. https://doi.org/10.1016/j.jocn.2018.03.026
Zhang W, Cui L, Wang W, Jiao Y, Zhang Y, Jiao J (2018) Early identification of anti-NMDA receptor encephalitis presenting cerebral lesions in unconventional locations on magnetic resonance imaging. J Neuroimmunol 320:101–106. https://doi.org/10.1016/j.jneuroim.2018.03.015
Li W, Wu S, Meng Q et al (2018) Clinical characteristics and short-term prognosis of LGI1 antibody encephalitis: a retrospective case study. BMC Neurol 18(1):96. https://doi.org/10.1186/s12883-018-1099-z
Probasco JC, Solnes L, Nalluri A et al (2017) Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm 4(4):e352. https://doi.org/10.1212/NXI.0000000000000352
Moreno-Ajona D, Prieto E, Grisanti F et al (2020) 18F-FDG-PET Imaging patterns in autoimmune encephalitis: impact of image analysis on the results. Diagnostics (Basel) 10(6):356. https://doi.org/10.3390/diagnostics10060356
Yin L, Han Y, Miao G, Jiang L, Xie S, Liu B (2018) CSF leukocyte, polykaryocyte, protein and glucose: their cut-offs of judging whether post-neurosurgical bacterial meningitis has been cured. Clin Neurol Neurosurg 174:198–202. https://doi.org/10.1016/j.clineuro.2018.09.023
Hrishi AP, Sethuraman M (2019) Cerebrospinal Fluid (CSF) Analysis and interpretation in neurocritical care for acute neurological conditions. Indian J Crit Care Med 23(Suppl 2):S115–S119. https://doi.org/10.5005/jp-journals-10071-23187
Breiner A, Moher D, Brooks J, Cheng W, Hegen H, Deisenhammer F, McCudden CR, Bourque PR (2019) Adult CSF total protein upper reference limits should be age-partitioned and significantly higher than 0.45 g/L: a systematic review. J Neurol 266(3):616–624. https://doi.org/10.1007/s00415-018-09174-z
Blinder T, Lewerenz J (2019) Cerebrospinal fluid findings in patients with autoimmune encephalitis—a systematic analysis. Front Neurol 10:804. https://doi.org/10.3389/fneur.2019.00804
Liu CY, Zhu J, Zheng XY, Ma C, Wang X (2017) Anti-N-methyl-D-aspartate receptor encephalitis: a severe, potentially reversible autoimmune encephalitis. Mediators Inflamm 2017:6361479. https://doi.org/10.1155/2017/6361479
Graus F, Saiz A, Dalmau J (2020) GAD antibodies in neurological disorders—insights and challenges. Nat Rev Neurol 16(7):353–365. https://doi.org/10.1038/s41582-020-0359-x
Ariño H, Armangué T, Petit-Pedrol M et al (2016) Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology 87(8):759–765. https://doi.org/10.1212/WNL.0000000000003009
Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R, Rosenfeld MR, Lynch D, Graus F, Dalmau J (2014) Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 13(2):167–177. https://doi.org/10.1016/S1474-4422(13)70282-5. Erratum in: Lancet Neurol. 2014;13(2):135
Daif A, Lukas RV, Issa NP, Javed A, VanHaerents S, Reder AT, Tao JX, Warnke P, Rose S, Towle VL, Wu S (2018) Antiglutamic acid decarboxylase 65 (GAD65) antibody-associated epilepsy. Epilepsy Behav 80:331–336. https://doi.org/10.1016/j.yebeh.2018.01.021
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. The study was approved by the University of Arkansas IRB.
Informed consent
The need for an informed consent was waved by the University of Arkansas IRB because this is a restrospective chart review study. No data with identifying information is used in the study.
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The manuscript has not been published and is not in consideration for publication in any other journal or for pre-publication. The corresponding author takes full responsibility for the data, the analyses and interpretation, and the conduct of the study. All authors had full access to all of the data. I have the right to publish any and all data separate and apart from any sponsor.
Rights and permissions
About this article
Cite this article
Elkhider, H., Sharma, R., Kapoor, N. et al. Autoimmune encephalitis and seizures, cerebrospinal fluid, imaging, and EEG findings: a case series. Neurol Sci 43, 2669–2680 (2022). https://doi.org/10.1007/s10072-021-05617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05617-0