Abstract
Objective
To determine whether erenumab is effective and safe in refractory chronic migraine with medication overuse headache.
Methods
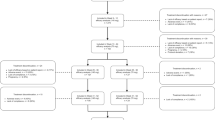
In this prospective, multicentric, real-life study, chronic migraine with medication overuse headache patients who received erenumab were recruited. Study inclusion was limited to patients who previously failed onabotulinumtoxinA in addition to at least three other pharmacological commonly used migraine preventive medication classes.
Results
Of 396 patients who received erenumab, 38% (n = 149) met inclusion criteria. After 3 months, 51% (n = 76) and 20% (n = 30) patients achieved ≥ 50% and ≥ 75% reduction in monthly headache days, respectively. Monthly pain medications intake decreased from 46.1 ± 35.3 to 16.8 ± 13.9 (p < 0.001), while monthly headache days decreased from 25.4 ± 5.4 to 14.1 ± 8.6 (p < 0.001). Increasing efficacy of erenumab over the study period was observed. Allodynia was a negative predictive factor of erenumab response (odds ratio = 0.47; p = 0.03). Clinical conversion to episodic migraine with no medication overuse was observed in 64% (n = 96) patients. No serious adverse events were observed.
Conclusions
Erenumab reduced significantly migraine frequency and pain medication intake in refractory chronic migraine with MOH patients.




Similar content being viewed by others
Data availability
The authors take full responsibility for the data, the analysis, and interpretation of the research, and they have full access to all of the data.
References
Natoli JL, Manack A, Dean B et al (2010) Global prevalence of chronic migraine: a systematic review. Cephalalgia 30:599–609
Munksgaard SB, Jensen RH (2014) Medication overuse headache. Headache 54:1251–1257
Lanteri-Minet M, Duru G, Mudge M, Cottrell S (2011) Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia 31:837–850
D’Amico D, Leone M, Grazzi L, Bussone G (2008) When should “chronic migraine” patients be considered “refractory” to pharmacological prophylaxis? Neurol Sci 29(Suppl 1):S55–S58
Schulman EA, Brahin EJ (2008) Refractory headache: historical perspective, need, and purposes for an operational definition. Headache 48:770–777
Schulman EA, Lake AE 3rd, Goadsby PJ et al (2008) Defining refractory migraine and refractory chronic migraine: proposed criteria from the Refractory Headache Special Interest Section of the American Headache Society. Headache 48:778–782
Sacco S, Braschinsky M, Ducros A et al (2020) European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine & Headache Alliance (EMHA). J Headache Pain 21:76
(2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211
Urits I, Jones MR, Gress K et al (2019) CGRP antagonists for the treatment of chronic migraines: a comprehensive review. Curr Pain Headache Rep 23:29
Tepper S, Ashina M, Reuter U et al (2017) Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16:425–434
Goadsby PJ, Reuter U, Hallstrom Y et al (2017) A controlled trial of erenumab for episodic migraine. N Engl J Med 377:2123–2132
Barbanti P, Aurilia C, Egeo G, Fofi L, Cevoli S, Colombo B, Filippi M, Frediani F, Bono F, Grazzi L, Salerno A, Mercuri B, Carnevale A, Altamura C, Vernieri F (2021) Erenumab in the prevention of high-frequency episodic and chronic migraine: Erenumab in real life in Italy (EARLY), the first Italian multicenter, prospective real-life study. Headache 61(2):363–372. https://doi.org/10.1111/head.14032
Tepper SJ (2019) CGRP and headache: a brief review. Neurol Sci 40:99–105
Matteo E, Favoni V, Pascazio A, Pensato U, Benini M, Asioli GM, Merli E, Calabrò C, Cortelli P, Pierangeli G, Cevoli S (2020) Erenumab in 159 high frequency and chronic migraine patients: real-life results from the Bologna headache center. Neurol Sci 41(2):483–484. https://doi.org/10.1007/s10072-020-04667-0
Lambru G, Hill B, Murphy M, Tylova I, Andreou AP (2020) A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain 21:61
Ornello R, Casalena A, Frattale I et al (2020) Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain 21:32
Curone M, Tullo V, Bussone G (2020) Effectiveness of erenumab in chronic migraine patients with associated medication overuse headache: a prospective observational study. Neurol Sci 41:509–510
Scheffler A, Messel O, Wurthmann S et al (2020) Erenumab in highly therapy-refractory migraine patients: first German real-world evidence. J Headache Pain 21:84
Cainazzo MM, Baraldi C, Ferrari A, Lo Castro F, Pani L, Guerzoni S (2021) Erenumab for the preventive treatment of chronic migraine complicated with medication overuse headache: an observational, retrospective, 12-month real-life study. Neurol Sci. https://doi.org/10.1007/s10072-021-05105-5
Shin HE, Park JW, Kim YI, Lee KS (2008) Headache Impact Test-6 (HIT-6) scores for migraine patients: Their relation to disability as measured from a headache diary. J Clin Neurol 4:158–163
Sarchielli P, Pini LA, Zanchin G et al (2006) Clinical-biochemical correlates of migraine attacks in rizatriptan responders and non-responders. Cephalalgia 26:257–265
Bendtsen L, Sacco S, Ashina M et al (2018) Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European Headache Federation. J Headache Pain 19:91
Ornello R, Tiseo C, Frattale I et al (2019) The appropriate dosing of erenumab for migraine prevention after multiple preventive treatment failures: a critical appraisal. J Headache Pain 20:99
Dodick DW, Ashina M, Brandes JL et al (2018) ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 38:1026–1037
Reuter U, Goadsby PJ, Lanteri-Minet M et al (2018) Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 392:2280–2287
Raffaelli B, Kalantzis R, Mecklenburg J et al (2020) Erenumab in chronic migraine patients who previously failed five first-line oral prophylactics and onabotulinumtoxinA: a dual-center retrospective observational study. Front Neurol 11:417
Pensato U, Favoni V, Pascazio A, Benini M, Asioli GM, Merli E, Calabrò C, Cortelli P, Pierangeli G, Cevoli S (2020) Erenumab efficacy in highly resistant chronic migraine: a real-life study. Neurol Sci 41(2):457–459. https://doi.org/10.1007/s10072-020-04658-1
Russo A, Silvestro M, Scotto di Clemente F et al (2020) Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain 21:69
Pellesi L, Do TP, Ashina H, Ashina M, Burstein R (2020) Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache 60:1056–1065
Tepper SJ, Diener HC, Ashina M et al (2019) Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology 92:e2309–e2320
Sacco S, Bendtsen L, Ashina M et al (2019) European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain 20:6
Lipton RB, Burstein R, Buse DC, Dodick DW, Koukakis R, Klatt J, Cheng S, Chou DE (2021) Efficacy of erenumab in chronic migraine patients with and without ictal allodynia. Cephalalgia 3331024211010305. https://doi.org/10.1177/03331024211010305
Ashkenazi A, Sholtzow M, Shaw JW, Burstein R, Young WB (2007) Identifying cutaneous allodynia in chronic migraine using a practical clinical method. Cephalalgia 27:111–117
Seo JG, Park SP (2019) Clinical significance of sensory hypersensitivities in migraine patients: does allodynia have a priority on it? Neurol Sci 40:393–398
Lovati C, Giani L, Mele F et al (2016) Brain plasticity and migraine transformation: fMRI evidences. Expert Rev Neurother 16:1413–1425
Barbanti P, Aurilia C, Egeo G, Fofi L (2019) Erenumab: from scientific evidence to clinical practice-the first Italian real-life data. Neurol Sci 40:177–179
Schwedt TJ (2018) Preventive therapy of migraine Continuum (Minneap Minn) 24:1052–1065
Deen M, Correnti E, Kamm K et al (2017) Blocking CGRP in migraine patients - a review of pros and cons. J Headache Pain 18:96
Ashina M, Goadsby PJ, Reuter U et al (2019) Long-term safety and tolerability of erenumab: Three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia 39:1455–1464
Acknowledgements
We would like to thank Cecilia Baroncini who edited the English text.
Author information
Authors and Affiliations
Contributions
UP and CB analyzed the data and drafted the manuscript; VF, MMC, PT, AP, DM, EM, SQ, GMA, PC, GP, SG, and SC acquired and analyzed data, and critically revised the manuscript. SC conceived and designed the study.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by an independent ethics committee or local institutional review board at each participating site, and written informed consent was obtained from all enrolled patients. All clinical investigations were conducted according to the latest version of the Declarations of Helsinki.
Consent to participate
Written informed consent was collected from the patients for the inclusion of deidentified clinical data in a scientific publication, in accordance with the Declaration of Helsinki.
Consent for publication
All authors agreed with this final version.
Conflict of interest
Carlo Baraldi and Simona Guerzoni received travel grants and honorary from Allergen, Novartis, Teva, and Ely Lilly. Maria Michela Cainazzo received travel grants and honorary from Allergen, Novartis, IBSA, and Ely Lilly. Sabina Cevoli received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Novartis, Teva, Lilly, Allergan, Ibsa, and Lundbeck. Valentina Favoni received honoraria as a speaker or for participating in advisory boards from Ely-Lilly, Novartis, and Teva. The other authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pensato, U., Baraldi, C., Favoni, V. et al. Real-life assessment of erenumab in refractory chronic migraine with medication overuse headache. Neurol Sci 43, 1273–1280 (2022). https://doi.org/10.1007/s10072-021-05426-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05426-5