Abstract
Objective
So far, a limited number of real-world evidence studies about the effectiveness and safety of alemtuzumab (ALM) have been published, some of them with a relatively small number of included patients. We aimed to study the efficacy and safety of ALM in real-world clinical practice in two MS centers in Slovenia and Croatia.
Methods
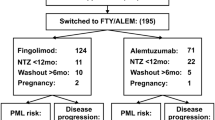
This was a retrospective chart review of 71 consecutive patients with relapsing–remitting MS who were treated with ALM from 2015 till 2018. The following data were collected: gender, age at disease onset, disease duration at ALM initiation, previous disease modifying therapy, number of relapses, active MRI lesions, and EDSS in the year prior to ALM initiation and every year of follow-up.
Results
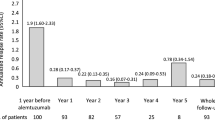
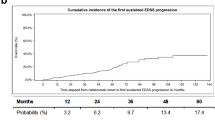
All patients completed the standard dosing schedule and were followed for a mean time of 3.2±1.1 years after the initiation of treatment. Complete data for the 2 years after treatment (relapses, EDSS, and MRI) were available for 48 patients, of which 14 (29.2%) achieved NEDA. Clinical NEDA was achieved in 38 out of 63 participants (60.3%). In year 1, 24 out of 57 (42.1%) patients achieved NEDA. In year 2, 26 out of 41 (63.4%) patients achieved NEDA. Lower EDSS prior to starting ALM was the only independent predictor of NEDA in a multivariable model. Adverse events occurred in 58 participants (84.1%), with no new safety signals identified.
Conclusion
According to the data from our cohort of early active RRMS patients we conclude ALM efficacy remains high in the real-world clinical practice.

Similar content being viewed by others
References
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Miller T, Fisher E, Sandbrink R, Lake SL, Margolin DH, Oyuela P, Panzara MA, Compston DA, CARE-MS II investigators (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380:1829–1839
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA, CARE-MS I investigators (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380:1819–1828
Rao SP, Sancho J, Campos-Rivera J, Boutin PM, Severy PB, Weeden T, Shankara S, Roberts BL, Kaplan JM (2012) Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS One 7:e39416
Ruck T, Bittner S, Wiendl H, Meuth SG (2015) Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci 16:16414–16439
McCall B (2019) Alemtuzumab to be restricted pending review. says EMA. Lancet 393:1683
Ziemssen T, Hillert J, Butzkueven H (2016) The importance of collecting structured clinical information on multiple sclerosis. BMC Med 14:81
Willis MD, Harding KE, Pickersgill TP, Wardle M, Pearson OR, Scolding NJ, Smee J, Robertson NP (2016) Alemtuzumab for multiple sclerosis: long term follow-up in a multi-centre cohort. Mult Scler 22:1215–1223
Prosperini L, Annovazzi P, Boffa L, Buscarinu MC, Gallo A, Matta M, Moiola L, Musu L, Perini P, Avolio C, Barcella V, Bianco A, Farina D, Ferraro E, Pontecorvo S, Granella F, Grimaldi LME, Laroni A, Lus G, Patti F, Pucci E, Pasca M, Sarchielli P (2018) Italian Alemtuzumab Study Group. No evidence of disease activity (NEDA-3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36-month real-world study. J Neurol 265:2851–2860
Huhn K, Bayas A, Doerck S, Frank B, Gerbershagen K, Hellwig K, Kallmann B, Kleinschnitz C, Kleiter I, Lee DH, Limmroth V, Mäurer M, Meuth S, Rieckmann P, Ruck T, Gold R, Linker RA (2018) Alemtuzumab as rescue therapy in a cohort of 50 relapsing-remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol 265:1521–1527
Frau J, Coghe G, Lorefice L, Fenu G, Musu L, Cocco E (2019) Efficacy and safety of alemtuzumab in a real-life cohort of patients with multiple sclerosis. J Neurol 266:1405–1411
Zmira O, Halpern AI, Abraham L, Achiron A (2020) Efficacy and safety of alemtuzumab treatment in a real-world cohort of patients with multiple sclerosis. Acta Neurol Belg. https://doi.org/10.1007/s13760-020-01375-6
di Ioia M, Di Stefano V, Farina D, Di Tommaso V, Travaglini D, Pietrolongo E, Sensi SL, Onofrj M, De Luca G (2020) Alemtuzumab treatment of multiple sclerosis in real-world clinical practice: a report from a single Italian center. Mult Scler Relat Disord 38:101504
https://www.ema.europa.eu/en/medicines/human/EPAR/lemtrada#product-information-section. Accessed 21 July 2020
Šega-Jazbec S, Barun B, Horvat Ledinek A, Fabekovac V, Krbot Skorić M, Habek M (2017) Management of infusion related reactions associated with alemtuzumab in patients with multiple sclerosis. Mult Scler Relat Disord 17:151–153
Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung HP, Havrdova E, Schippling S, Selmaj KW, Traboulsee A, Compston DAS, Margolin DH, Thangavelu K, Chirieac MC, Jody D, Xenopoulos P, Hogan RJ, Panzara MA, Arnold DL, CARE-MS II (2017) CAMMS03409 Investigators. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 89:1117–1126
Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, Wilkins A, Pearson O, Ziemssen T, Hutchinson M, McGuigan C, Jokubaitis V, Spelman T, Horakova D, Havrdova E, Trojano M, Izquierdo G, Lugaresi A, Prat A, Girard M, Duquette P, Grammond P, Alroughani R, Pucci E, Sola P, Hupperts R, Lechner-Scott J, Terzi M, Van Pesch V, Rozsa C, Grand'Maison F, Boz C, Granella F, Slee M, Spitaleri D, Olascoaga J, Bergamaschi R, Verheul F, Vucic S, McCombe P, Hodgkinson S, Sanchez-Menoyo JL, Ampapa R, Simo M, Csepany T, Ramo C, Cristiano E, Barnett M, Butzkueven H, Coles A (2017) MSBase Study Group. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 16:271–281
Filippi M, Preziosa P, Meani A, Ciccarelli O, Mesaros S, Rovira A, Frederiksen J, Enzinger C, Barkhof F, Gasperini C, Brownlee W, Drulovic J, Montalban X, Cramer SP, Pichler A, Hagens M, Ruggieri S, Martinelli V, Miszkiel K, Tintorè M, Comi G, Dekker I, Uitdehaag B, Dujmovic-Basuroski I, Rocca MA (2018) Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol 17(2):133–142
Berger T, Elovaara I, Fredrikson S, McGuigan C, Moiola L, Myhr KM, Oreja-Guevara C, Stoliarov I, Zettl UK (2017) Alemtuzumab use in clinical practice: recommendations from European multiple sclerosis experts. CNS Drugs 31:33–50
Author information
Authors and Affiliations
Contributions
Study concept and design: Brecl Jakob, Habek. Acquisition of data: Brecl Jakob, Barun, Gomezelj, Gabelić, Šega Jazbec, Adamec, Horvat Ledinek, Rot, Krbot Skorić, Habek.
Analysis and interpretation of data: Brecl Jakob, Barun, Gomezelj, Gabelić, Šega Jazbec, Adamec, Horvat Ledinek, Rot, Krbot Skorić, Habek. Drafting of the manuscript: Brecl Jakob, Gomezelj, Krbot Skorić, Habek. Critical revision of the manuscript for important intellectual content: Brecl Jakob, Barun, Gomezelj, Gabelić, Šega Jazbec, Adamec, Horvat Ledinek, Rot, Krbot Skorić, Habek. Administrative, technical, and material support: Brecl Jakob, Gomezelj, Krbot Skorić, Habek.
Corresponding author
Ethics declarations
Conflict of interest
GBJ: participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Novartis, Pliva/Teva, Roche.
BB: participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals.
SG: participated as a clinical investigator and/or received speaker fees from: Roche.
TG: participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
SSJ: participated as a clinical investigator and/or received consultation and/or speaker fees from: Bayer, Biogen, Sanofi Genzyme, Merck, Novartis, Pliva/Teva, Roche.
IA: participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
AHL: participated as a clinical investigator and/or received consultation and/or speaker fees from: Bayer, Biogen, Sanofi Genzyme, Merck, Novartis, Pliva/Teva, Roche.
UR: participated as a clinical investigator and/or received consultation and/or speaker fees from: Bayer, Biogen, Sanofi Genzyme, Merck, Novartis, Pliva/Teva, Roche.
MKS: received consultation and/or speaker fees from: Sanofi Genzyme, Roche.
MH: participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Brecl Jakob, G., Barun, B., Gomezelj, S. et al. Effectiveness and safety of alemtuzumab in the treatment of active relapsing–remitting multiple sclerosis: a multicenter, observational study. Neurol Sci 42, 4591–4597 (2021). https://doi.org/10.1007/s10072-021-05145-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05145-x