Abstract
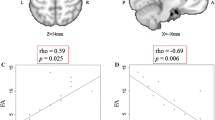
The diagnosis of amyotrophic lateral sclerosis (ALS) requires both upper and lower motor neuron signs. However, quite a few patients with ALS lack the upper motor neuron sign during the disease. This study sought to investigate whether metabolites, including glutamate (Glu), N-acetyl aspartate (NAA), and gamma aminobutyric acid (GABA), in the supplementary motor area (SMA) measured by magnetic resonance spectroscopy (MRS), could be a surrogate biomarker for ALS. Twenty-five patients with ALS and 12 controls underwent 3.0-T MR scanning, which measured Glu, NAA, and GABA. Finally, receiver operating characteristic (ROC) curves were created and the area under curve (AUC) was calculated to assess the diagnostic power. Logistic regression analysis revealed the usefulness of both Glu and NAA for the differentiation of ALS from controls (Glu, P = 0.009; NAA, P = 0.033). The ratio of Glu to NAA or GABA was significantly increased in patients with ALS (Glu/NAA, P = 0.027; Glu/GABA, P = 0.003). Both the AUCs were more than 0.7, with high specificity but low sensitivity. The present findings might indicate that both the Glu/NAA and the Glu/GABA ratios in the SMA could be potential biomarkers for the diagnosis of ALS.



Similar content being viewed by others
References
Riku Y, Atsuta N, Yoshida M, Tatsumi S, Iwasaki Y, Mimuro M, Watanabe H, Ito M, Senda J, Nakamura R, Koike H, Sobue G (2014) Differential motor neuron involvement in progressive muscular atrophy: a comparative study with amyotrophic lateral sclerosis. BMJ Open 4(5):e005213
Novotny EJ Jr, Fulbright RK, Pearl PL, Gibson KM, Rothman DL (2003) Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol 54(Suppl 6):S25–S31
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955
Bradley WG, Bowen BC, Pattany PM, Rotta F (1999) 1H-magnetic resonance spectroscopy in amyotrophic lateral sclerosis. J Neurol Sci 169(1-2):84–86
Pioro EP, Majors AW, Mitsumoto H, Nelson DR, Ng TC (1999) 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology 53(1):71–79
Bowen BC, Pattany PM, Bradley WG, Murdoch JB, Rotta F, Younis AA, Duncan RC, Quencer RM (2000) MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 21(4):647–658
Pohl C, Block W, Karitzky J, Traber F, Schmidt S, Grothe C et al (2001) Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis. Arch Neurol 58(5):729–735
Schuff N, Rooney WD, Miller R, Gelinas DF, Amend DL, Maudsley AA, Weiner MW (2001) Reanalysis of multislice (1)H MRSI in amyotrophic lateral sclerosis. Magn Reson Med 45(3):513–516
Rule RR, Suhy J, Schuff N, Gelinas DF, Miller RG, Weiner MW (2004) Reduced NAA in motor and non-motor brain regions in amyotrophic lateral sclerosis: a cross-sectional and longitudinal study. Amyotroph Lateral Scler Other Motor Neuron Disord 5(3):141–149
Wang S, Poptani H, Woo JH, Desiderio LM, Elman LB, McCluskey LF et al (2006) Amyotrophic lateral sclerosis: diffusion-tensor and chemical shift MR imaging at 3.0 T. Radiology 239(3):831–838
Mitsumoto H, Ulug AM, Pullman SL, Gooch CL, Chan S, Tang MX et al (2007) Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology 68(17):1402–1410
Unrath A, Ludolph AC, Kassubek J (2007) Brain metabolites in definite amyotrophic lateral sclerosis. A longitudinal proton magnetic resonance spectroscopy study. J Neurol 254(8):1099–1106
Sivak S, Bittsansky M, Kurca E, Turcanova-Koprusakova M, Grofik M, Nosal V et al (2010) Proton magnetic resonance spectroscopy in patients with early stages of amyotrophic lateral sclerosis. Neuroradiology 52(12):1079–1085
Verma G, Woo JH, Chawla S, Wang S, Sheriff S, Elman LB, McCluskey LF, Grossman M, Melhem ER, Maudsley AA, Poptani H (2013) Whole-brain analysis of amyotrophic lateral sclerosis by using echo-planar spectroscopic imaging. Radiology 267(3):851–857
Han J, Ma L (2010) Study of the features of proton MR spectroscopy ((1)H-MRS) on amyotrophic lateral sclerosis. J Magn Reson Imaging 31(2):305–308
Foerster BR, Callaghan BC, Petrou M, Edden RA, Chenevert TL, Feldman EL (2012) Decreased motor cortex gamma-aminobutyric acid in amyotrophic lateral sclerosis. Neurology 78(20):1596–1600
Foerster BR, Pomper MG, Callaghan BC, Petrou M, Edden RA, Mohamed MA et al (2013) An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol 70(8):1009–1016
Foerster BR, Carlos RC, Dwamena BA, Callaghan BC, Petrou M, Edden RA et al (2014) Multimodal MRI as a diagnostic biomarker for amyotrophic lateral sclerosis. Ann Clin Transl Neurol 1(2):107–114
Sako W, Abe T, Izumi Y, Harada M, Kaji R (2016) The ratio of N-acetyl aspartate to glutamate correlates with disease duration of amyotrophic lateral sclerosis. J Clin Neurosci 27:110–113
Atassi N, Xu M, Triantafyllou C, Keil B, Lawson R, Cernasov P, Ratti E, Long CJ, Paganoni S, Murphy A, Salibi N, Seethamraju R, Rosen B, Ratai EM (2017) Ultra high-field (7tesla) magnetic resonance spectroscopy in amyotrophic lateral sclerosis. PLoS One 12(5):e0177680
Cheong I, Marjanska M, Deelchand DK, Eberly LE, Walk D, Oz G (2017) Ultra-high field proton MR spectroscopy in early-stage amyotrophic lateral sclerosis. Neurochem Res 42(6):1833–1844
Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC (2011) Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry 82(5):476–486
Sako W, Abe T, Izumi Y, Harada M, Kaji R (2016) Fractional anisotropy in the supplementary motor area correlates with disease duration and severity of amyotrophic lateral sclerosis. Neurol Sci 37(4):573–577
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1):273–289
Sako W, Fujita K, Vo A, Rucker JC, Rizzo JR, Niethammer M, Carbon M, Bressman SB, Uluğ AM, Eidelberg D (2015) The visual perception of natural motion: abnormal task-related neural activity in DYT1 dystonia. Brain 138(Pt 12):3598–3609
Sako W, Abe T, Izumi Y, Yamazaki H, Matsui N, Harada M, Kaji R (2017) Spontaneous brain activity in the sensorimotor cortex in amyotrophic lateral sclerosis can be negatively regulated by corticospinal fiber integrity. Neurol Sci 38(5):755–760
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541
Lowe MJ, Mock BJ, Sorenson JA (1998) Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 7(2):119–132
Zang Y, Jiang T, Lu Y, He Y, Tian L (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22(1):394–400
Block W, Karitzky J, Traber F, Pohl C, Keller E, Mundegar RR et al (1998) Proton magnetic resonance spectroscopy of the primary motor cortex in patients with motor neuron disease: subgroup analysis and follow-up measurements. Arch Neurol 55(7):931–936
Cheong I, Deelchand DK, Eberly LE, Marjańska M, Manousakis G, Guliani G, Walk D, Öz G (2019) Neurochemical correlates of functional decline in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 90(3):294–301
Fiszman ML, Ricart KC, Latini A, Rodriguez G, Sica RE (2010) In vitro neurotoxic properties and excitatory aminoacids concentration in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Relationship with the degree of certainty of disease diagnoses. Acta Neurol Scand 121(2):120–126
Milanese M, Giribaldi F, Melone M, Bonifacino T, Musante I, Carminati E, Rossi PIA, Vergani L, Voci A, Conti F, Puliti A, Bonanno G (2014) Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 64:48–59
Rothstein JD, Martin LJ, Kuncl RW (1992) Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med 326(22):1464–1468
Sage CA, Peeters RR, Gorner A, Robberecht W, Sunaert S (2007) Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 34(2):486–499
Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Munte TF (2009) Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp Neurol 217(1):147–153
Keil C, Prell T, Peschel T, Hartung V, Dengler R, Grosskreutz J (2012) Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci 13:141
Zhang J, Yin X, Zhao L, Evans AC, Song L, Xie B, Li H, Luo C, Wang J (2014) Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. J Neurol 261(2):412–421
Corcia P, Tauber C, Vercoullie J, Arlicot N, Prunier C, Praline J, Nicolas G, Venel Y, Hommet C, Baulieu JL, Cottier JP, Roussel C, Kassiou M, Guilloteau D, Ribeiro MJ (2012) Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS One 7(12):e52941
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65(1):1–105
Acknowledgements
We would like to thank all the subjects for joining this study.
Funding
This study was supported by the Uehara Memorial Foundation, Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 20K12670, and Grants-in Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and informed consent statement
All subjects provided written informed consent, following detailed explanation of the procedures, and the study was approved by the local ethics committee of Tokushima University Hospital. This study was performed according to the Declaration of Helsinki.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure 1.
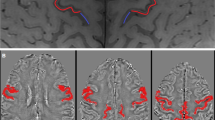
Differences in FA and ReHo, and results of correlation analyses. There were no significant differences in FA and ReHo between groups (A). ALS, amyotrophic lateral sclerosis; Ctr, control; FA value was positively correlated with ReHo. FA, fractional anisotropy; ReHo, regional homogeneity. (PNG 90 kb)
Rights and permissions
About this article
Cite this article
Sako, W., Izumi, Y., Abe, T. et al. MR spectroscopy and imaging-derived measurements in the supplementary motor area for biomarkers of amyotrophic lateral sclerosis. Neurol Sci 42, 4257–4263 (2021). https://doi.org/10.1007/s10072-021-05107-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05107-3