Abstract
Rationale
The activation of the glucagon-like peptide-1 receptor (GLP-1R) has been purported to have antidepressant-like and cognitive-enhancing effects. Many people suffering from major depressive disorder (MDD) also experience deficits in cognition. While currently approved antidepressant pharmacotherapies can alleviate the mood symptoms in some patients, they do not treat the cognitive ones.
Objectives
We tested whether systemic administration of a GLP-1R agonist would alter location discrimination, a cognitive task that is diminished in humans with MDD.
Methods
Male and female laboratory mice (6–8 weeks old, N = 6–14/sex) were trained in a touchscreen operant task of location discrimination. Upon reaching baseline criterion, mice were administered vehicle or a GLP-1R agonist, Exendin-4, systemically prior to testing in probe trials of varying difficulty.
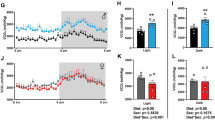
Results
Following GLP-1R activation, males showed modest yet non-significant performance in the location discrimination task. Females, however, showed enhanced performance during the most difficult probe tests following Exendin-4 administration.
Conclusions
GLP-1R activation appears to enhance overall performance in the location discrimination task and does so in a sex- and difficulty-dependent manner. These preliminary yet impactful data indicate that GLP-1R agonists may be useful as an adjunctive pharmacotherapy to treat cognitive deficits associated with MDD and/or multiple neurological disorders.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article as well as from the corresponding author upon reasonable request.
References
Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012) The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32(14):4812–4820. https://doi.org/10.1523/JNEUROSCI.6326-11.2012
Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E (2013) The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38(8):1259–1270. https://doi.org/10.1016/j.psyneuen.2012.11.009
Graham DL, Erreger K, Galli A, Stanwood GD (2013) GLP-1 analog attenuates cocaine reward. Mol Psychiatry 18(9):961–962. https://doi.org/10.1038/mp.2012.141
Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, Perez E, Miller J, Hoffer BJ, Greig NH, Pick CG (2013) Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol 239:170–182. https://doi.org/10.1016/j.expneurol.2012.10.001
During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9(9):1173–1179. https://doi.org/10.1038/nm919
Huang HJ, Chen YH, Liang KC, Jheng YS, Jhao JJ, Su MT, Lee-Chen GJ, Hsieh-Li HM (2012) Exendin-4 protected against cognitive dysfunction in hyperglycemic mice receiving an intrahippocampal lipopolysaccharide injection. PLoS One 7(7):e39656. https://doi.org/10.1371/journal.pone.0039656
Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF 3rd, Becker JT (2006) Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry 14(5):419–427. https://doi.org/10.1097/01.JGP.0000203130.45421.69
Weitz ES, Hollon SD, Twisk J, van Straten A, Huibers MJ, David D, DeRubeis RJ, Dimidjian S, Dunlop BW, Cristea IA, Faramarzi M, Hegerl U, Jarrett RB, Kheirkhah F, Kennedy SH, Mergl R, Miranda J, Mohr DC, Rush AJ, Segal ZV, Siddique J, Simons AD, Vittengl JR, Cuijpers P (2015) Baseline depression severity as moderator of depression outcomes between cognitive behavioral therapy vs pharmacotherapy: an individual patient data meta-analysis. JAMA Psychiatry 72(11):1102–1109. https://doi.org/10.1001/jamapsychiatry.2015.1516
McDermott LM, Ebmeier KP (2009) A meta-analysis of depression severity and cognitive function. J Affect Disord 119(1–3):1–8. https://doi.org/10.1016/j.jad.2009.04.022
Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A (2016) Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry 3(5):425–435. https://doi.org/10.1016/S2215-0366(16)00012-2
Isacson R, Nielsen E, Dannaeus K, Bertilsson G, Patrone C, Zachrisson O, Wikstrom L (2011) The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol 650(1):249–255. https://doi.org/10.1016/j.ejphar.2010.10.008
Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, Wikstrom L (2008) Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 86(2):326–338. https://doi.org/10.1002/jnr.21483
Hunter K, Holscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13:33. https://doi.org/10.1186/1471-2202-13-33
Moller C, Sommer W, Thorsell A, Rimondini R, Heilig M (2002) Anxiogenic-like action of centrally administered glucagon-like peptide-1 in a punished drinking test. Prog Neuro-Psychopharmacol Biol Psychiatry 26(1):119–122
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10(6):434–445. https://doi.org/10.1038/nrn2639
Chaves Filho AJM, Cunha NL, de Souza AG, Soares MV, Juca PM, de Queiroz T, Oliveira JVS, Valvassori SS, Barichello T, Quevedo J, de Lucena D, Macedo DS (2020) The GLP-1 receptor agonist liraglutide reverses mania-like alterations and memory deficits induced by D-amphetamine and augments lithium effects in mice: relevance for bipolar disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 99:109872. https://doi.org/10.1016/j.pnpbp.2020.109872
Bierman EJ, Comijs HC, Jonker C, Beekman AT (2005) Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry 13(8):686–693. https://doi.org/10.1176/appi.ajgp.13.8.686
Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS (2005) Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 43(5):659–674. https://doi.org/10.1016/j.neuropsychologia.2004.09.002
Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325(5937):210–213. https://doi.org/10.1126/science.1173215
Yassa MA, Stark CE (2011) Pattern separation in the hippocampus. Trends Neurosci 34(10):515–525. https://doi.org/10.1016/j.tins.2011.06.006
Fujii T, Saito DN, Yanaka HT, Kosaka H, Okazawa H (2014) Depressive mood modulates the anterior lateral CA1 and DG/CA3 during a pattern separation task in cognitively intact individuals: a functional MRI study. Hippocampus 24(2):214–224. https://doi.org/10.1002/hipo.22216
Graham DL, Durai HH, Trammell T, Noble BL, Mortlock DP, Galli A, Stanwood GD (2020) A novel mouse model of glucagon-like peptide-1 receptor expression: a look at the brain. J Comp Neurol 528:2445–2470. https://doi.org/10.1002/cne.24905
Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, Saksida LM (2013) The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc 8(10):2006–2021. https://doi.org/10.1038/nprot.2013.124
Rock PL, Roiser JP, Riedel WJ, Blackwell AD (2014) Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 44(10):2029–2040. https://doi.org/10.1017/S0033291713002535
Ledford H (2016) Drugmakers target depression’s cognitive fog. Nature 530(7588):17. https://doi.org/10.1038/530017a
Yagi S, Galea LAM (2019) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44(1):200–213. https://doi.org/10.1038/s41386-018-0208-4
Jonasson Z (2005) Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev 28(8):811–825. https://doi.org/10.1016/j.neubiorev.2004.10.006
Yagi S, Chow C, Lieblich SE, Galea LA (2016) Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus 26(1):87–101. https://doi.org/10.1002/hipo.22493
Park J, Shin GI, Park YM, Kim IY, Jang DP (2017) Sex differences of cognitive load effects on object-location binding memory. Biomed Eng Lett 7(4):305–309. https://doi.org/10.1007/s13534-017-0038-z
Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19(14):5792–5801
Lagace DC, Fischer SJ, Eisch AJ (2007) Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus 17(3):175–180. https://doi.org/10.1002/hipo.20265
Tzeng WY, Chen LH, Cherng CG, Tsai YN, Yu L (2014) Sex differences and the modulating effects of gonadal hormones on basal and the stressor-decreased newly proliferative cells and neuroblasts in dentate gyrus. Psychoneuroendocrinology 42:24–37. https://doi.org/10.1016/j.psyneuen.2014.01.003
Bangasser DA, Eck SR, Telenson AM, Salvatore M (2018) Sex differences in stress regulation of arousal and cognition. Physiol Behav 187:42–50. https://doi.org/10.1016/j.physbeh.2017.09.025
Rajab E, Alqanbar B, Naiser MJ, Abdulla HA, Al-Momen MM, Kamal A (2014) Sex differences in learning and memory following short-term dietary restriction in the rat. Int J Dev Neurosci 36:74–80. https://doi.org/10.1016/j.ijdevneu.2014.05.011
Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A (2012) Exendin-4 decreases amphetamine-induced locomotor activity. Physiol Behav 106(4):574–578. https://doi.org/10.1016/j.physbeh.2012.03.014
Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R (2008) Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149(10):4768–4777. https://doi.org/10.1210/en.2008-0180
Brown E, Cuthbertson DJ, Wilding JP (2018) Newer GLP-1 receptor agonists and obesity-diabetes. Peptides 100:61–67. https://doi.org/10.1016/j.peptides.2017.12.009
Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, Reiner DJ, Hahn JD, Hayes MR, Kanoski SE (2017) A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry 23:1555–1565. https://doi.org/10.1038/mp.2017.91
Moser MB, Moser EI (1998) Functional differentiation in the hippocampus. Hippocampus 8(6):608–619. https://doi.org/10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7
Substance Abuse and Mental Health Services (2013) Results from the 2012 National Survey on Drug Use and Health: mental health findings. NSDUH Series H-47, HHS Publication No. (SMA) 13–4805. Rockville, MD
Acknowledgments
We would like to thank Matt Croxall (Lafayette Instruments) for technical advice.
Funding
This work was supported by National Institutes of Health grant R03MH110749 (DLG), the Florida State University Council on Research & Creativity (DLG), and the Florida State University College of Medicine.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by DLG and GDS. TST, NLH, HSM, and DLG performed the experiments. Data analyses were performed by TST and DLG. Manuscript was written by DLG, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All protocols were approved by the local Institutional Animal Care and Use Committee, and all studies were performed in accordance with the recommendations in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Informed consent
None
Consent to participate
N/A
Consent for publication
All authors have read and approved of the manuscript and its publication.
Code availability
N/A
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trammell, T.S., Henderson, N.L., Madkour, H.S. et al. GLP-1R activation alters performance in cognitive tasks in a sex-dependent manner. Neurol Sci 42, 2911–2919 (2021). https://doi.org/10.1007/s10072-020-04910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04910-8