Abstract
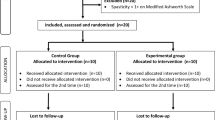
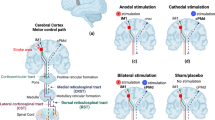
Spasticity is a common symptom in stroke survivors. This study is double-blinded, sham-controlled randomized, clinical trial with three parallel arms. The aim of the study was to investigate the effects of anodal trans-cranial direct current stimulation (a-tDCS) over the damaged primary motor cortex (M1) on spasticity of the wrist flexor and also the activity of wrist flexor and extensor muscles in sub-acute stroke patients. This study was performed on 32 stroke patients. The patients are assigned to three groups (intervention, sham, and control). All participants in the first two groups received 20-min concurrent M1 a-tDCS or sham tDCS and functional electrical stimulation (FES) for 10 sessions (5 sessions per week), while participants in control group were given only 20-min FES for 10 sessions. Modified Ashworth scale of wrist flexors and also electromyography (EMG) activity of flexor carpi radialis (FCR) and extensor carpi radialis (ECR) were recorded before, immediately, and 1 month after the interventions. A significant reduction was shown in the MAS and EMG activity of FCR muscle at passive rest position of the wrist, immediately and 1 month after the intervention in M1 a-tDCS compared to sham and control groups (p < 0.001). Also, the EMG activity of FCR and ECR muscles during active wrist flexion and extension increased immediately and 1 month after intervention in M1 a-tDCS compared to the other groups, respectively (p < 0.001). M1 a-tDCS can significantly decrease the spasticity of wrist flexor muscle and also increase the wrist flexor and extensor muscles activity in stroke patients during active flexion and extension.





Similar content being viewed by others
References
Sohn MK, Jee SJ, Kim YW (2013) Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann Rehabil Med 37:759–765
Hesse S, Werner C, Schonhardt E, Bardeleben A, Jenrich W, Kirker S (2007) Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci 25:9–15
Elsner B, Kugler J, Pohl M, Mehrholz J (2016) Transcranial direct current stimulation for improving spasticity after stroke: a systematic review with meta-analysis. J Rehabil Med 48:565–570
Zorowitz RD, Gillard PJ, Brainin M (2013) Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology 80:S45–S52
Li S (2017) Spasticity, motor recovery, and neural plasticity after stroke. Front Neurol 8:120
Triccas LT, Burridge JH, Hughes A-M, Pickering RM, Desikan M, Rothwell JC, Verheyden G (2016) Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin Neurophysiol 127:946–955
Sunnerhagen KS (2016) Predictors of spasticity after stroke. Curr Phys Med Rehabil Rep 4:182–185
Viana R, Laurentino G, Souza R, Fonseca J, Silva Filho E, Dias S, Teixeira-Salmela L, Monte-Silva K (2014) Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: a pilot randomized controlled trial. NeuroRehabilitation 34:437–446
Kwakkel G, Meskers CG (2015) Botulinum toxin A for upper limb spasticity. Lancet Neurol 14:969–971
Naumann M, Albanese A, Heinen F, Molenaers G, Relja M (2006) Safety and efficacy of botulinum toxin type A following long-term use. Eur J Neurol 13:35–40
Shaw L, Rodgers H, Price C, van Wijck F, Shackley P, Steen N, Barnes M, Ford G, Graham L (2010) BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol Assess 14:1–113
Kaji R, Osako Y, Suyama K, Maeda T, Uechi Y, Iwasaki M (2010) Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. J Neurol 257:1330–1337
Taricco M, Adone R, Pagliacci C, Telaro E (2000) Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev
Albright AL, Cervi A, Singletary J (1991) Intrathecal baclofen for spasticity in cerebral palsy. Jama 265:1418–1422
Feldman RG, Kelly-Hayes M, Conomy JP, Foley JM (1978) Baclofen for spasticity in multiple sclerosis double-blind crossover and three-year study. Neurology 28:1094
Lannin NA, Novak I, Cusick A (2007) A systematic review of upper extremity casting for children and adults with central nervous system motor disorders. Clin Rehabil 21:963–976
Yan T, Hui-Chan CW, Li LS (2005) Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke 36:80–85
Sabut SK, Sikdar C, Kumar R, Mahadevappa M (2011) Functional electrical stimulation of dorsiflexor muscle: effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation 29:393–400
Rothwell JC. Control of human voluntary movement: Springer Science & Business Media; 2012.
Krause P, Szecsi J, Straube A (2008) Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil 22:627–634
Krassioukov A, Warburton DE, Teasell R, Eng JJ, Team SCIRER (2009) A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 90:682–695
Ralston KE, Harvey LA, Batty J, Lee BB, Ben M, Cusmiani R, Bennett J (2013) Functional electrical stimulation cycling has no clear effect on urine output, lower limb swelling, and spasticity in people with spinal cord injury: a randomised cross-over trial. J Phys 59:237–243
Nowak DA, Grefkes C, Ameli M, Fink GR (2009) Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair 23:641–656
Del Felice A, Daloli V, Masiero S, Manganotti P (2016) Contralesional cathodal versus dual transcranial direct current stimulation for decreasing upper limb spasticity in chronic stroke individuals: a clinical and neurophysiological study. J Stroke Cerebrovasc Dis 25:2932–2941
Koh C-L, Lin J-H, Jeng J-S, Huang S-L, Hsieh C-L (2017) Effects of Transcranial direct current stimulation with sensory modulation on stroke motor rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil 98:2477–2484
Auvichayapat N, Amatachaya A, Auvichayapat P (2014) Reduction of spasticity in cerebral palsy by anodal transcranial direct current stimulation. J Med Assoc Thail 97:954–962
Khedr EM, Shawky OA, El-Hammady DH, Rothwell JC, Darwish ES, Mostafa OM, Tohamy AM (2013) Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil Neural Repair 27:592–601
Bakheit A, Maynard V, Curnow J, Hudson N, Kodapala S (2003) The relation between Ashworth scale scores and the excitability of the α motor neurones in patients with post-stroke muscle spasticity. J Neurol Neurosurg Psychiatry 74:646–648
Albani G, BE SV, Bar D, Campanelli L, BE RG, Mauro A (2010) Use of surface EMG for evaluation of upper limb spasticity during botulinum toxin therapy in stroke patients. Funct Neurol 25:103
Fruhauf AMA, Politti F, Dal Corso S, Costa GC, da Conceição TA, Silva SM, Corrêa JCF, Corrêa FI (2017) Immediate effect of transcranial direct current stimulation combined with functional electrical stimulation on activity of the tibialis anterior muscle and balance of individuals with hemiparesis stemming from a stroke. J Phys Ther Sci 29:2138–2146
Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F (2011) A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14:1133–1145
Gandiga PC, Hummel FC, Cohen LG (2006) Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117:845–850
Thorsen R, Spadone R, Ferrarin M (2001) A pilot study of myoelectrically controlled FES of upper extremity. IEEE Trans Neural Syst Rehabil Eng 9:161–168
Alaerts K, Heremans E, Swinnen SP, Wenderoth N (2009) How are observed actions mapped to the observer’s motor system? Influence of posture and perspective. Neuropsychologia 47:415–422
Li R, Hu XL, Tong K, editors. Combined Electromyography (EMG)-driven system with functional electrical stimulation (FES) for poststroke rehabilitation. 2008 2nd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics; 2008: IEEE.
Allison G, Marshall R, Singer K (1993) EMG signal amplitude normalization technique in stretch-shortening cycle movements. J Electromyogr Kinesiol 3:236–244
Liu J, Ying D, Rymer WZ (2015) EMG burst presence probability: a joint time–frequency representation of muscle activity and its application to onset detection. J Biomech 48:1193–1197
Hu B, Zhang X, Mu J, Wu M, Wang Y (2018) Spasticity assessment based on the Hilbert–Huang transform marginal spectrum entropy and the root mean square of surface electromyography signals: a preliminary study. Biomed Eng Online 17:27
Charalambous CP (2014) Interrater reliability of a modified Ashworth scale of muscle spasticity. Classic Papers in Orthopaedics: Springer. p. 415-417.
George MS, Aston-Jones G (2010) Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology 35:301–316
Lo H-C, Tsai K-H, Su F-C, Chang G-L, Yeh C-Y (2009) Effects of a functional electrical stimulation-assisted leg-cycling wheelchair on reducing spasticity of patients after stroke. J Rehabil Med 41:242–246
Masoudian N, Ehsani F, Nazari M, Zoghi M, Jaberzadeh S (2020) Does M1 anodal transcranial direct current stimulation affects online and offline motor learning in patients with multiple sclerosis? Neurol Sci:1–8
Kaski D, Dominguez R, Allum J, Islam A, Bronstein A (2014) Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: a pilot randomized controlled study. Clin Rehabil 28:1115–1124
Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ (2014) Anodal transcranial direct current stimulation alters elbow flexor muscle recruitment strategies. Brain Stimul 7:443–450
Tanaka S, Hanakawa T, Honda M, Watanabe K (2009) Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res 196:459–465
Tanaka S, Takeda K, Otaka Y, Kita K, Osu R, Honda M, Sadato N, Hanakawa T, Watanabe K (2011) Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabil Neural Repair 25:565–569
Roche N, Lackmy A, Achache V, Bussel B, Katz R (2011) Effects of anodal transcranial direct current stimulation over the leg motor area on lumbar spinal network excitability in healthy subjects. J Physiol 589:2813–2826
Fujiwara T (2020) Mini-review article: the role of spinal reciprocal inhibition and intracortical inhibition in functional recovery from stroke. Exp Brain Res 238:1701–1705
Martins A (2016) The role of spasticity in functional neurorehabilitation–Part 1: the pathophysiology of spasticity, the relationship with neuroplasticity, spinal shock and clinical signs. Arch Medication 8:1–7
Wu D, Qian L, Zorowitz RD, Zhang L, Qu Y, Yuan Y (2013) Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: a randomized sham-controlled study. Arch Phys Med Rehabil 94:1–8
Acknowledgments
We would like to thank the Research Center of Neuromuscular Rehabilitation of the Semnan University of Medical Sciences and also the Clinical Research Development Unit of Kosar Educational and Research and Therapeutic Hospital of the Semnan University of Medical Sciences for cooperation and providing facilities for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Halakoo, S., Ehsani, F., Masoudian, N. et al. Does anodal trans-cranial direct current stimulation of the damaged primary motor cortex affects wrist flexor muscle spasticity and also activity of the wrist flexor and extensor muscles in patients with stroke?: a Randomized Clinical Trial. Neurol Sci 42, 2763–2773 (2021). https://doi.org/10.1007/s10072-020-04858-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04858-9