Abstract
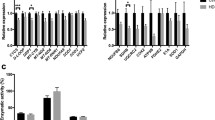
Huntington’s disease (HD) is a neurodegenerative disorder caused by a CAG nucleotide expansion, which encodes the amino acid glutamine, in the huntingtin gene. HD is characterized by motor, cognitive, and psychiatric dysfunctions. In a previous study, we showed by qPCR that some genes altered in an HD mouse model were also altered in blood of HD patients. These alterations were mainly with respect to the dynein family. Therefore, this study aimed to investigate whether dynein light chain Tctex type 1 (DYNLT1) is altered in HD patients and if there is a correlation between DYNLT1 gene expression changes and disease progression. We assessed the DYNLT1 gene expression in the blood of 19 HD patients and 20 healthy age-matched controls. Also, in 6 of these patients, we analyzed the DYNLT1 expression at two time points, 3 years apart. The DYNLT1 gene expression in the whole blood of HD patients was significantly downregulated and this difference was widened in later stages. These data suggest that DYNLT1 could emerge as a peripheral prognostic indicator in HD and, also, might be a target for potential intervention in the future.


Similar content being viewed by others
References
Aronin N, Chase K, Young C, Sapp E, Schwarz C, Matta N, Kornreich R, Lanwehrmeyer B, Bird E, Beal MF, Vonsattel JP, Smith T, Carraway R, Boyce FM, Young AB, Penney JB, DiFiglia M (1995) CAG expansion affects the expression of mutant huntingtin in the Huntington’s disease brain. Neuron 15:1193–1201. https://doi.org/10.1016/0896-6273(95)90106-X
Sharp AH, Loev SJ, Schilling G, Li SH, Li XJ, Bao J, Wagster MV, Kotzuk JA, Steiner JP, Lo A, Hedreen J, Sisodia S, Snyder SH, Dawson TM, Ryugo DK, Ross CA (1995) Widespread expression of Huntington’s disease gene (IT15) protein product. Neuron 14:1065–1074. https://doi.org/10.1016/0896-6273(95)90345-3
MacDonald ME, Bates GP, Buckler AJ et al (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 72:971–983. https://doi.org/10.1016/0092-8674(93)90585-E
Rawlins MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJW, Smeeth L (2016) The prevalence of huntington’s disease. Neuroepidemiology 46:144–153. https://doi.org/10.1159/000443738
Group HS (1996) Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord 11:136–142
Ribeiro FM, DeVries RA, Hamilton A et al (2014) Metabotropic glutamate receptor 5 knockout promotes motor and biochemical alterations in a mouse model of Huntington’s disease. Hum Mol Genet 23:2030–2042. https://doi.org/10.1093/hmg/ddt598
Paschal BM, Shpetner HS, Vallee RB (1987) MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol 105:1273–1282. https://doi.org/10.1083/jcb.105.3.1273
Ravikumar B, Acevedo-arozena A, Imarisio S et al (2005) Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet 37:771–776. https://doi.org/10.1038/ng1591
Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol 11:1680–1685. https://doi.org/10.1016/S0960-9822(01)00531-0
Moncke-Buchner E (2002) Counting CAG repeats in the Huntington’s disease gene by restriction endonuclease EcoP15I cleavage. Nucleic Acids Res 30:83e. https://doi.org/10.1093/nar/gnf082
Chevalier-Larsen ELFH (2006) Axonal transport and neurodegenerative diseases. Biochim Biophys Acta Gen Subj 1762:1094–1108. https://doi.org/10.1016/B978-008045046-9.00714-2
Vallee RB, Williams JC, Varma D, Barnhart LE (2004) Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol 58:189–200. https://doi.org/10.1002/neu.10314
Caviston JP, Ross JL, Antony SM, Tokito M, Holzbaur ELF (2007) Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc Natl Acad Sci U S A 104:10045–10050. https://doi.org/10.1073/pnas.0610628104
Melo TQ, D’Unhao AM, Martins SA et al (2013) Rotenone-dependent changes of anterograde motor protein expression and mitochondrial mobility in brain areas related to neurodegenerative diseases. Cell Mol Neurobiol 33:327–335. https://doi.org/10.1007/s10571-012-9898-z
Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ (2008) N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci 28:2783–2792. https://doi.org/10.1523/JNEUROSCI.0106-08.2008
Day CL, Puthalakath H, Skea G et al (2004) Localization of dynein light chains 1 and 2 and their pro-apoptotic ligands. Biochem J 377:597–605. https://doi.org/10.1042/bj20031251
Puorro G, Marsili A, Sapone F, Pane C, de Rosa A, Peluso S, de Michele G, Filla A, Saccà F (2018) Peripheral markers of autophagy in polyglutamine diseases. Neurol Sci 39:149–152. https://doi.org/10.1007/s10072-017-3156-6
Capiluppi E, Romano L, Rebora P, Nanetti L, Castaldo A, Gellera C, Mariotti C, Macerollo A, Cislaghi MG (2020) Late-onset Huntington’s disease with 40–42 CAG expansion. Neurol Sci 41:869–876. https://doi.org/10.1007/s10072-019-04177-8
Areal LB, Ribeiro FM, Muniz MR et al (2017) Role of dynein axonemal heavy chain 6 gene expression as a possible biomarker for Huntington’s disease: a translational study. J Mol Neurosci 63:342–348. https://doi.org/10.1007/s12031-017-0984-z
Castilhos RM, Augustin MC, Santos JA, Perandones C, Saraiva-Pereira ML, Jardim LB, on Behalf of Rede Neurogenética (2016) Genetic aspects of Huntington’s disease in Latin America. A systematic review. Clin Genet 89:295–303. https://doi.org/10.1111/cge.12641
Brocklebank D, Gayán J, Andresen JM, Roberts SA, The International-Venezuela Collaborative Research Group, Young AB, Snodgrass SR, Penney JB, Ramos-Arroyo MA, Cha JJ, Rosas HD, Hersch SM, Feigin A, Cherny SS, Wexler NS, Housman DE, Cardon LR (2009) Repeat instability in the 27-39 CAG range of the HD gene in the Venezuelan Kindreds: counseling implications. Am J Med Genet B Neuropsychiatr Genet 150:425–429. https://doi.org/10.1002/ajmg.b.30826
Pillai JA, Hansen LA, Masliah E, Goldstein JL, Edland SD, Corey-Bloom J (2012) Clinical severity of Huntington’s disease does not always correlate with neuropathologic stage. Mov Disord 27:1099–1103. https://doi.org/10.1002/mds.25026
Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M, The Predict-HD Investigators and Coordinators of the Huntington Study Group (2008) Detection of Huntington’s disease decades before diagnosis: the predict-HD study. J Neurol Neurosurg Psychiatry 79:874–880. https://doi.org/10.1136/jnnp.2007.128728
Acknowledgments
The authors thank the Health Sciences Center facility Laboratório de Análises Biomoleculares (LABIOM) for the support with molecular analysis. We are grateful to the laboratory Hermes Pardini, for molecular diagnosis.
Funding
This work was supported by Fundação de Amparo a Pesquisa do Estado do Espírito Santo (FAPES) grants to DAMG, FAPES/CNPq/MS-Decit/SESA – PPSUS (edital n° 10/2013), process 65823648/2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were in accordance with the Federal University of Espírito Santo Human Research Committee by the number 1.856.340/2016.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Rosseto, S.M., Alarcon, T.A., Rocha, D.M.C. et al. DYNLT1 gene expression is downregulated in whole blood of patients at different Huntington’s disease stages. Neurol Sci 42, 1963–1967 (2021). https://doi.org/10.1007/s10072-020-04772-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04772-0