Abstract
Background and purpose
Diagnosis of Parkinson’s disease (PD) cognitive impairment at early stages is challenging compared to the stage of PD dementia where functional impairment is apparent and easily diagnosed. Hence, to evaluate potential early stage cognitive biomarkers, we assessed frontal lobe metabolic alterations using in vivo multi-voxel proton magnetic resonance spectroscopic imaging (1H-MRSI).
Method
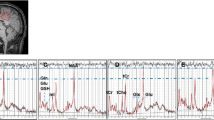
Frontal metabolism was studied in patients with PD with normal cognition (PD-CN) (n = 26), with cognitive impairment (PD-CI) (n = 27), and healthy controls (HC) (n = 30) using a single slice (two-dimensional) 1H-MRSI at 3 T. The acquired spectra were post-processed distinctly for voxels corresponding to the bilateral middle/superior frontal gray matter (GM) and frontal white matter (WM) regions (delineated employing neuromorphometrics atlas) using the LC-Model software.
Result
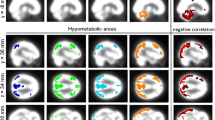
Significant (post hoc p < 0.016) reduction in the concentration of N-acetyl aspartate (NAA) in the middle and superior frontal GMs and total choline (tCho) and total creatine (tCr) in the frontal WM was observed in PD-CI compared to PD-CN and HC, while that in HC and PD-CN groups were comparable. The NAA and tCr/tCho metabolite concentrations showed significant (p < 0.05) positive correlations with cognitive test scores in the frontal GM and WM, respectively. The receiver operating curve (ROC) analysis revealed significant (p < 0.05) “area under curve” for NAA/tNAA in the frontal GM and tCho in the frontal WM.
Conclusion
The frontal metabolic profile is altered in cognitively impaired PD compared with cognitively normal PD. Neuronal function loss (NAA), altered energy metabolism (Cr), and cholinergic (Cho) neural transmission are implicated in PD cognitive pathology. Frontal neuro-metabolism may promisingly serve as PD cognitive biomarker.




Similar content being viewed by others
Data availability
Data are available as supplementary material.
References
Lopiano L, Modugno N, Marano P, Sensi M, Meco G, Cannas A, Gusmaroli G, Tamma F, Mancini F, Quatrale R, Costanzo AM, Gualberti G, Melzi G, di Luzio Paparatti U, Antonini A (2016) Motor outcomes in patients with advanced Parkinson’s disease treated with levodopa/carbidopa intestinal gel in Italy: an interim analysis from the GREENFIELD observational study. Neurol Sci 37:1785–1792. https://doi.org/10.1007/s10072-016-2664-0
Goldman JG, Vernaleo BA, Camicioli R et al (2018) Cognitive impairment in Parkinson’s disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Parkinsons Dis 4:19. https://doi.org/10.1038/s41531-018-0055-3
Aarsland D, Kvaløy JT, Andersen K, Larsen JP, Tang MX, Lolk A, Kragh-Sørensen P, Marder K (2007) The effect of age of onset of PD on risk of dementia. J Neurol 254:38–45. https://doi.org/10.1007/s00415-006-0234-8
Lucetti C, Del Dotto P, Gambaccini G et al (2001) Proton magnetic resonance spectroscopy (1H-MRS) of motor cortex and basal ganglia in de novo Parkinson’s disease patients. Neurol Sci 22:69–70. https://doi.org/10.1007/s100720170051
Prell T (2018) Structural and functional brain patterns of non-motor syndromes in Parkinson’s disease. Front Neurol 9:138. https://doi.org/10.3389/fneur.2018.00138
Pagonabarraga J, Gómez-Ansón B, Rotger R, Llebaria G, García-Sánchez C, Pascual-Sedano B, Gironell A, Delfino M, Ruscalleda J, Kulisevsky J (2012) Spectroscopic changes associated with mild cognitive impairment and dementia in Parkinson’s disease. Dement Geriatr Cogn Disord 34:312–318. https://doi.org/10.1159/000345537
Almuqbel M, Melzer TR, Myall DJ, MacAskill MR, Pitcher TL, Livingston L, Wood KL, Keenan RJ, Dalrymple-Alford JC, Anderson TJ (2016) Metabolite ratios in the posterior cingulate cortex do not track cognitive decline in Parkinson’s disease in a clinical setting. Parkinsonism Relat Disord 22:54–61. https://doi.org/10.1016/j.parkreldis.2015.11.008
Gratwicke J, Jahanshahi M, Foltynie T (2015) Parkinson’s disease dementia: a neural networks perspective. Brain. 138:1454–1476. https://doi.org/10.1093/brain/awv104
Tal A, Kirov II, Grossman RI, Gonen O (2012) The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed 25:1392–1400. https://doi.org/10.1002/nbm.2812
Chaudhary S, Kumaran SS, Kaloiya GS, Goyal V, Sagar R, Kalaivani M, Jaganathan NR, Mehta N, Srivastava A (2020) Domain specific cognitive impairment in Parkinson’s patients with mild cognitive impairment. J Clin Neurosci 75:99–105. https://doi.org/10.1016/j.jocn.2020.03.015
Calabresi P, Galletti F, Saggese E, Ghiglieri V, Picconi B (2007) Neuronal networks and synaptic plasticity in Parkinson’s disease: beyond motor deficits. Parkinsonism Relat Disord 13:S259–S262. https://doi.org/10.1016/S1353-8020(08)70013-0
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679. https://doi.org/10.1002/mrm.1910300604
Griffith HR, den Hollander JA, Okonkwo OC, O’Brien T, Watts RL, Marson DC (2008) Brain metabolism differs in Alzheimer’s disease and Parkinson’s disease dementia. Alzheimers Dement 4:421–427. https://doi.org/10.1016/j.jalz.2008.04.008
Provencher SW (2019) LCModel & LCMgui User’s Manual
Penny W, Friston K, Ashburner J, Kiebel S, Nichols T (2007) Statistical parametric mapping. Elsevier. https://doi.org/10.1016/B978-0-12-372560-8.X5000-1
Asman AJ, Chambless LB, Thompson RC, Landman BA (2013) Out-of-atlas likelihood estimation using multi-atlas segmentation. Med Phys 40:043702. https://doi.org/10.1118/1.4794478
Doelken MT, Mennecke A, Stadlbauer A, Kecskeméti L, Kasper BS, Struffert T, Doerfler A, Stefan H, Hammen T (2010) Multi-voxel magnetic resonance spectroscopy at 3T in patients with idiopathic generalised epilepsy. Seizure. 19:485–492. https://doi.org/10.1016/j.seizure.2010.07.005
Robbins TW, Cools R (2014) Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov Disord 29:597–607. https://doi.org/10.1002/mds.25853
Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA (2009) The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 132:2958–2969. https://doi.org/10.1093/brain/awp245
Trojano L, Papagno C (2018) Cognitive and behavioral disorders in Parkinson’s disease: an update. II: behavioral disorders. Neurol Sci 39:53–61. https://doi.org/10.1007/s10072-017-3155-7
Bohnen NI, Albin RL (2011) The cholinergic system and Parkinson disease. Behav Brain Res 221:564–573. https://doi.org/10.1016/j.bbr.2009.12.048
Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG (2015) A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 9:23. https://doi.org/10.3389/fnsys.2015.00023
Li W, Qin W, Liu H, Fan L, Wang J, Jiang T, Yu C (2013) Subregions of the human superior frontal gyrus and their connections. Neuroimage. 78:46–58. https://doi.org/10.1016/j.neuroimage.2013.04.011
Su L, Blamire AM, Watson R, He J, Hayes L, O’Brien JT (2016) Whole-brain patterns of 1H-magnetic resonance spectroscopy imaging in Alzheimer’s disease and dementia with Lewy bodies. Transl Psychiatry 6:e877–e877. https://doi.org/10.1038/tp.2016.140
Klietz M, Bronzlik P, Nösel P, Wegner F, Dressler DW, Dadak M, Maudsley AA, Sheriff S, Lanfermann H, Ding XQ (2019) Altered neurometabolic profile in early Parkinson’s disease: a study with short echo-time whole brain MR spectroscopic imaging. Front Neurol 10:777. https://doi.org/10.3389/fneur.2019.00777
Riese F, Gietl A, Zölch N, Henning A, O’Gorman R, Kälin AM, Leh SE, Buck A, Warnock G, Edden RAE, Luechinger R, Hock C, Kollias S, Michels L (2015) Posterior cingulate γ-aminobutyric acid and glutamate/glutamine are reduced in amnestic mild cognitive impairment and are unrelated to amyloid deposition and apolipoprotein E genotype. Neurobiol Aging 36:53–59. https://doi.org/10.1016/j.neurobiolaging.2014.07.030
Xu H, Zhang H, Zhang J, Huang Q, Shen Z, Wu R (2016) Evaluation of neuron-glia integrity by in vivo proton magnetic resonance spectroscopy: implications for psychiatric disorders. Neurosci Biobehav Rev 71:563–577. https://doi.org/10.1016/j.neubiorev.2016.09.027
Gómez-Ansón B, Alegret M, Muñoz E, Sainz A, Monte GC, Tolosa E (2007) Decreased frontal choline and neuropsychological performance in preclinical Huntington disease. Neurology. 68:906–910. https://doi.org/10.1212/01.wnl.0000257090.01107.2f
Lindner M, Bell T, Iqbal S, Mullins PG, Christakou A (2017) In vivo functional neurochemistry of human cortical cholinergic function during visuospatial attention. PLoS One 12:e0171338. https://doi.org/10.1371/journal.pone.0171338
Müller MLTM, Bohnen NI (2013) Cholinergic dysfunction in Parkinson’s disease. Curr Neurol Neurosci Rep 13:377. https://doi.org/10.1007/s11910-013-0377-9
Chung W-S, Welsh CA, Barres BA, Stevens B (2015) Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci 18:1539–1545. https://doi.org/10.1038/nn.4142
Heinrichs-Graham E, Santamaria PM, Gendelman HE, Wilson TW (2017) The cortical signature of symptom laterality in Parkinson’s disease. NeuroImage Clin 14:433–440. https://doi.org/10.1016/j.nicl.2017.02.010
Boecker H, Ceballos-Baumann AO, Volk D, Conrad B, Forstl H, Haussermann P (2007) Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol 64(7):984–988. https://doi.org/10.1001/archneur.64.7.984
Acknowledgements
SC acknowledges Fellowship from SERB (SB/CT/046/2013) and AIIMS. JC Bose fellowship from SERB is acknowledged by NRJ.
Funding
This research work did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SC participated in data acquisition, processing, analysis, and manuscript preparation. SSK participated in MRI protocol designing, execution, analysis, and manuscript preparation. VG helped in diagnosing patients with Parkinson’s disease and patient recruitment. GSK helped in cognitive evaluation of subjects. MK helped in statistical analysis. NRJ helped in concept development and discussion. RS helped in cognitive evaluation of subjects; NM helped in concept and discussion. AS helped in participant recruitment.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest regarding the publication of the work.
Ethics approval
The study is approved by the institutional ethics committee (approval number IECPG-503/21.09.2016).
Consent to participate
A written informed consent has been obtained from the participants prior to the commencement of study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, S., Kumaran, S.S., Goyal, V. et al. Frontal lobe metabolic alterations characterizing Parkinson’s disease cognitive impairment. Neurol Sci 42, 1053–1064 (2021). https://doi.org/10.1007/s10072-020-04626-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04626-9