Abstract
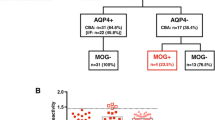
The differential diagnosis between acquired inflammatory demyelinating syndromes of the central nervous system (CNS), such as multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD) and acute disseminated encephalomyelitis (ADEM) can be very challenging at onset. Apart from cerebrospinal fluid oligoclonal bands and anti-aquaporin-4 antibodies (AQP4-Ab), definite diagnostic biomarkers are lacking. Anti-myelin oligodendrocyte glycoprotein antibodies (MOG-Abs) have been increasingly described in children with AQP4-seronegative NMOSD, ADEM and other inflammatory demyelinating CND syndromes; despite partial overlaps with AQP4-Ab disease, a novel “MOG-Ab-disorder” phenotype has been suggested. In this study, we tested the presence of MOG-Ab and AQP4-Ab in 57 children at first onset of acute neurological symptoms; three clinical subgroups were identified: 12 patients had acquired inflammatory demyelinating CNS syndromes, 11 had other autoimmune/immune-mediated disorders of the central and peripheral nervous system and 34 had non-immune-mediated CNS disorders. MOG-Abs were found positive only in a subset of cases in the subgroup with acquired inflammatory demyelinating CNS syndromes (in 2/12 patients, both with non-MS phenotype) and in none of the patients with other autoimmune and immune-mediated disorders of the central and peripheral nervous system or with non-immune-mediated disorders of the CNS.
Data from the literature review support clinical and analytical observations.
Similar content being viewed by others
References
Comabella M, Montalban X (2014) Body fluid biomarkers in multiple sclerosis. Lancet Neurol 13:113–126
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revision of the McDonald criteria. Lancet Neurol 17:162–173
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6:805–815
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364:2106–2112
Ramanathan S, Dale RC, Brilot F (2016) Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 15:307–324
Hacohen Y, Absoud M, Deiva K, Hemingway C, Nytrova P, Woodhall M, Palace J, Wassmer E, Tardieu M, Vincent A, Lim M, Waters P (2015) Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm 2:e81
Hennes EM, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, Brantner-Inthaler S, Diepold K, Eisenkölbl A, Gotwald T, Kuchukhidze G, Gruber-Sedlmayr U, Häusler M, Höftberger R, Karenfort M, Klein A, Koch J, Kraus V, Lechner C, Leiz S, Leypoldt F, Mader S, Marquard K, Poggenburg I, Pohl D, Pritsch M, Raucherzauner M, Schimmel M, Thiels C, Tibussek D, Vieker S, Zeches C, Berger T, Reindl M, Rostásy K, BIOMARKER Study Group (2017) Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 89:900–908
Hennes EM, Baumann M, Lechner C, Rostásy K (2018) MOG spectrum disorders and role of MOG-antibodies in clinical practice. Neuropediatrics 49:3–11
Peschl P, Bradl M, Höftberger R, Berger T, Reindl M (2017) Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol 8:1–15
Jarius S, Ruprecht K, Kleiter I et al (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation 13:279
Cobo-Calvo A, Ruiz A, Maillart E et al (2018) Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 90:1858–1869
O'Connor KC, McLaughlin KA, De Jager PL et al (2007) Self-antigen tetramers discriminate between myelin autoantibodies to native or denaturated protein. Nat Med 13:211–217
Jarius S, Paul F, Aktas O et al (2018) MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 15:134
Hacohen Y, Mankad K, Chong WK, Barkhof F, Vincent A, Lim M, Wassmer E, Ciccarelli O, Hemingway C (2017) Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology 89:269–278
Jurynczyk M, Geraldes R, Probert F, Woodhall MR, Waters P, Tackley G, DeLuca G, Chandratre S, Leite MI, Vincent A, Palace J (2017) Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain 140:617–627
Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E, International Pediatric Multiple Sclerosis Study Group (2013) International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 19:1261–1267
Teunissen CE, Petzold A, Bennett JL, Berven FS, Brundin L, Comabella M, Franciotta D, Frederiksen JL, Fleming JO, Furlan R, Hintzen RQ, Hughes SG, Johnson MH, Krasulova E, Kuhle J, Magnone MC, Rajda C, Rejdak K, Schmidt HK, van Pesch V, Waubant E, Wolf C, Giovannoni G, Hemmer B, Tumani H, Deisenhammer F (2009) A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73:1914–1922
Duignan S, Wright S, Rossor T, Cazabon J, Gilmour K, Ciccarelli O, Wassmer E, Lim M, Hemingway C, Hacohen Y (2018) Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev Med Child Neurol 60:958–962
Nosadini M, Granata T, Matricardi S, Freri E, Ragona F, Papetti L, Suppiej A, Valeriani M, Sartori S, Italian Working Group on Paediatric Anti-N-methyl-D-aspartate Receptor Encephalitis (2019) Relapse risk factors in anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol 61:1101–1107
Nosadini M, Toldo I, Tascini B, Bien CG, Parmeggiani L, De Gaspari P, Zuliani L, Sartori S (2019) LGI1 and CASPR2 autoimmunity in children: systematic literature review and report of a young girl with Morvan syndrome. J Neuroimmunol 335:577008
Sartori S, Priante E, Pettenazzo A, Marson P, Suppiej A, Benini F, Perilongo G, Toldo I (2014) Intrathecal synthesis of oligoclonal bands in rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome: new evidence supporting immunological pathogenesis. J Child Neurol 29:421–425
Ramanathan S, Mohammad S, Tantsis E et al (2018) Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 89:127–137
Hacohen Y, Palace J (2018) Time to separate MOG-Ab-associated disease from AQP4-Ab-positive neuromyelitis optica spectrum disorder. Neurology 90:947–948
Dos Passos GR, Oliveira LM, da Costa BK, Apostolos-Pereira SL, Callegaro D, Fujihara K, Sato DK (2018) MOG-IgG-associated optic neuritis, encephalitis, and myelitis: lessons learned from neuromyelitis optica spectrum disorder. Front Neurol 9:217
Jarius S, Ruprecht K, Kleiter I et al (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Parts 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 13:280
Zuliani L, Nosadini M, Gastaldi M, Spatola M, Iorio R, Zoccarato M, Mariotto S, De Gaspari P, Perini F, Ferrari S, Evoli A, Sartori S, Franciotta D, Giometto B (2019) Management of antibody-mediated autoimmune encephalitis in adults and children: literature review and consensus-based practical recommendations. Neurol Sci 40:2017–2030
Chen JJ, Flanagan EP, Jitprapaikulsan J et al (2018) Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophtalmol 195:8–15
Reindl M, Jarius S, Rostasy K, Berger T (2017) Myelin oligodendrocyte glycoprotein antibodies: how clinically useful are they? Curr Opin Neurol 30:295–301
Waters PJ, Komorowski L, Woodhall M, Lederer S, Majed M, Fryer J, Mills J, Flanagan EP, Irani SR, Kunchok AC, McKeon A, Pittock SJ (2019) A multicenter comparison of MOG-IgG cell-based assays. Neurology 92:e1250–e1255
Reindl M, Di Pauli F, Rostásy K, Berger T (2013) The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol 9:455–461
Wells E, Hacohen Y, Waldman A, Tillema JM, Soldatos A, Ances B, Benseler S, Bielekova B, Dale RC, Dalmau J, Gaillard W, Gorman M, Greenberg B, Hyslop A, Pardo CA, Tasker RC, Yeh EA, Bar-Or A, Pittock S, Vanderver A, Banwell B, attendees of the International Neuroimmune Meeting (2018) Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol 14:433–445
Acknowledgments
We thank Euroimmun AG a PerkinElmer company, Lübeck, Germany for kindly supplying CBA assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Musso, G., Nosadini, M., Gallo, N. et al. Possible clinical role of MOG antibody testing in children presenting with acute neurological symptoms. Neurol Sci 41, 2553–2559 (2020). https://doi.org/10.1007/s10072-020-04379-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04379-5