Abstract
Objective
To study compound muscle action potential (CMAP) decrement by low-frequency repetitive nerve stimulation (RNS) in different hand muscles of amyotrophic lateral sclerosis (ALS) patients and the relationship with split hand phenomenon and clinical manifestation.
Methods
Clinical and decrement data of 51 ALS patients who had done RNS in different hand muscles were retrospectively reviewed from November 2016 to July 2017. Decrement data of 24 myasthenia gravis (MG) and 20 Lambert Eaton myasthenia syndrome (LEMS) patients was also reviewed to compare decrement pattern with hand muscles of ALS patients.
Results
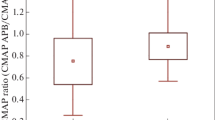
There was statistical significance between the decrement ratio of abductor digiti minimi (ADM) and abductor pollicis brevis (APB) as well as ADM and first dorsal interosseous (FDI). The decrements of the APB, ADM, and FDI were negatively correlated with their amplitude of CMAPs respectively. The difference between the decrement ratio of the APB and ADM was negatively correlated with the division ratio (CMAPAPB/CMAPADM). The decrement ratio of APB and FDI was negatively correlated with their muscle strength. There was a mild correlation between decrement ratio of APB and disease course. There was no statistical significance in the decrement pattern of the three-hand muscles of ALS patients. There was statistical significance in decrement pattern between APB of ALS and LEMS patients.
Conclusion
Dysfunction of neuromuscular transmission was found in hand muscles of ALS patients, APB was involved most significantly. The dysfunction of neuromuscular transmission might be involved in the formation of the split hand phenomenon.



Similar content being viewed by others
References
van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, van den Berg LH (2017) Amyotrophic lateral sclerosis. Lancet 390(10107):2084–2098. https://doi.org/10.1016/S0140-6736(17)31287-4
Kuwabara S, Sonoo M, Komori T, Hirashima F, Inaba A, Misawa S, Hatanaka Y, Tokyo Metropolitan Neuromuscular Electrodiagnosis Study Group (2008) Dissociated small hand muscle atrophy in amyotrophic lateral sclerosis: frequency, extent, and specificity. Muscle Nerve 37:426e30. https://doi.org/10.1002/mus.20949
Weber M, Eisen A, Stewart H, Hirota N (2000) The split hand in ALS has a cortical basis. J NeurolSci 180:66e70. https://doi.org/10.1016/S0022-510X(00)00430-5
Kanai K, Kuwabara S, Misawa S, Tamura N, Ogawara K, Nakata M, Sawai S, Hattori T, Bostock H (2006) Altered axonal excitability properties in amyotrophic lateral sclerosis: impaired potassium channel function related to disease stage. Brain 129:953e62. https://doi.org/10.1093/brain/awl024
Vucic S, Kiernan MC (2006) Axonal excitability properties in amyotrophic lateral sclerosis. Clin Neurophysiol 117:1458e66. https://doi.org/10.1016/j.clinph.2006.04.016
Schelhaas HJ, van de Warrenburg BP, Kremer HP, Zwarts MJ (2003) The “split hand” phenomenon: evidence of a spinal origin. Neurology 61:1619e20. https://doi.org/10.1212/01.WNL.0000096009.50213.6C
Park D, Park JS (2017) Terminal latency abnormality in amyotrophic lateral sclerosis without split hand syndrome. Neurol Sci 38(5):775–781. https://doi.org/10.1007/s10072-017-2842-8
Martineau É, Di Polo A, Vande Velde C, Robitaille R (2018) Dynamic neuromuscular remodeling precedes motor-unit loss in a mouse model of ALS. Elife 15(7):41973. https://doi.org/10.7554/eLife.41973.001
Tremblay E, Martineau É, Robitaille R (2017) Opposite synaptic alterations at the neuromuscular junction in an ALS mouse model: when motor units matter. J Neurosci 37(37):8901–8918. https://doi.org/10.1523/JNEUROSCI.3090-16.2017
Cappello V, Francolini M (2017) Neuromuscular junction dismantling in amyotrophic lateral sclerosis. Int J Mol Sci 18(10):2092. https://doi.org/10.3390/ijms18102092
Alanazy MH, Hegedus J, White C, Korngut L (2017) Decremental responses in patients with motor neuron disease. Brain Behav 26;7(11):e00846. https://doi.org/10.1002/brb3.846
Killian JM, Wilfong AA, Burnett L, Appel SH, Boland D (1994) Decremental motor responses to repetitive nerve stimulation in ALS. Muscle Nerve 17:747–754. https://doi.org/10.1002/mus.880170708
Henderson R, Baumann F, Hutchinson N, McCombe P (2009) CMAP decrement in ALS. Muscle Nerve 39:555–556. https://doi.org/10.1002/mus.21105
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders: official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases 1(5):293–9.
Luigetti M, Conte A, Del Grande A, Bisogni G, Romano A, Sabatelli M, Amin Lari A, Ghavanini AA, Bokaee HR (2012) Sural nerve pathology in ALS patients: a single-centre experience. Neurol Sci 33(5):1095–1099. https://doi.org/10.1007/s10072-011-0909-5
Simon NG, Lomen-Hoerth C, Kiernan MC (2014) Patterns of clinical and electrodiagnostic abnormalities in early amyotrophic lateral sclerosis. Muscle Nerve 50(6):894–899. https://doi.org/10.1002/mus.24244
Yang H, Liu M, Li X, Cui B, Fang J, Cui L (2015) Neurophysiological differences between flail arm syndrome and amyotrophic lateral sclerosis. PLoS One 9; 10(6):e0127601. https://doi.org/10.1371/journal.pone.0127601
Sun X, Zhang Z, Liu N (2016) Absence of split hand in the flail arm variant of ALS. Neurophysiol Clin 46(2):149–152. https://doi.org/10.1016/j.neucli.2016.03.002
Kim JE, Hong YH, Lee JH, Ahn SW, Kim SM, Park KS, Sung JJ, Lee KW, Seong SY (2015) Pattern difference of dissociated hand muscle atrophy in amyotrophic lateral sclerosis and variants. Muscle Nerve 51(3):333–337. https://doi.org/10.1002/mus.24323
Wang FC, De Pasqua V, Gerard P, Delwaide PJ (2001) Prognostic value of decremental responses to repetitive nerve stimulation in ALS patients. Neurology 57:897–899. https://doi.org/10.1212/WNL.57.5.897
Yamashita S, Sakaguchi H, Mori A, Kimura E, Maeda Y, Hirano T, Uchino M (2012) Significant CMAP decrement by repetitive nerve stimulation is more frequent in median than ulnar nerves of patients with amyotrophic lateral sclerosis. Muscle Nerve 45(3):426–428. https://doi.org/10.1002/mus.22301
Inoue K, Hemmi S, Miyaishi M, Kutoku Y, Murakami T, Kurokawa K, Sunada Y (2009) Muscular fatigue and decremental response to repetitive nerve stimulation in X-linked spinobulbar muscular atrophy. Eur J Neurol 16(1):76–80. https://doi.org/10.1111/j.1468-1331.2008.02349.x
Wadman RI, Vrancken AF, van den Berg LH, van der Pol WL (2012) Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Neurology 79(20):2050–2055. https://doi.org/10.1212/WNL.0b013e3182749eca
Iwanami T, Sonoo M, Hatanaka Y, Hokkoku K, Oishi C, Shimizu T (2011) Decremental responses to repetitive nerve stimulation (RNS) in motor neuron disease. Clin Neurophysiol 122(12):2530–2536. https://doi.org/10.1016/j.clinph.2011.05.019
Pera MC, Luigetti M, Pane M, Coratti G, Forcina N, Fanelli L, Mazzone ES, Antonaci L, Lapenta L, Palermo C, Ranalli D, Granata G, Lomonaco M, Servidei S, Mercuri E (2017) 6MWT can identify type 3 SMA patients with neuromuscular junction dysfunction. Neuromuscul Disord 27(10):879–882. https://doi.org/10.1016/j.nmd.2017.07.007
Baslo MB, Deymeer F, Serdaroglu P, Parman Y, Ozdemir C, Cuttini M (2006) Decrement pattern in Lambert-Eaton myasthenic syndrome is different from myasthenia gravis. Neuromuscul Disord 16(7):454–458. https://doi.org/10.1016/j.nmd.2006.05.009
Luigetti M, Modoni A, Lo Monaco M (2013) Low rate repetitive nerve stimulation in Lambert-Eaton myasthenic syndrome: peculiar characteristics of decremental pattern from a single-centre experience. Clin Neurophysiol 124(4):825–826. https://doi.org/10.1016/j.clinph.2012.08.026
Sanders DB (1993) Clinical neurophysiology of disorders of the neuromuscular junction. J Clin Neurophysiol 10:167–180
Acknowledgments
We thank the patients for their participation.
Contributions
(I) Conception and design: Y Zhao, C Yan, D Zhang; (II) Administrative support: C Yan; (III) Provision of study materials or patients: Y Zhao, D Zhang, L Cao; (IV) Collection and assembly of data: D Zhang, W Li, L Cao; (V) Data analysis and interpretation: D Zhang, L Cao, W Li, Y Zhao; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Funding
This study was supported by the Grants from National Natural Science Foundation of China (No.81701237).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, D., Zhao, Y., Yan, C. et al. CMAP decrement by low-frequency repetitive nerve stimulation in different hand muscles of ALS patients. Neurol Sci 40, 2609–2615 (2019). https://doi.org/10.1007/s10072-019-04027-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-04027-7