Abstract
Background
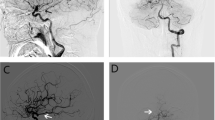
Posterior communicating artery (PcomA) aneurysm can be classified into sidewall or bifurcation types based on the anatomical variation of fetal-type posterior cerebral artery (fPCA). The aims of this study were to investigate the significance of fPCA as an independent risk factor for the rupture of PcomA aneurysm and to evaluate other associated morphological and clinical risk factors.
Methods
We retrospectively reviewed clinical and radiological findings of 255 patients with PcomA aneurysms, which were treated in a single tertiary institute between January 2009 and December 2016. Univariate and multivariate analyses were performed to evaluate the associations between morphological and clinical variables and rupture status. Subgroup analysis was also performed based on the aneurysms with and without fPCA.
Results
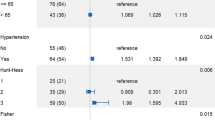
Fifty-five out of 255 PcomA aneurysms (21.6%) were associated with fPCA. Multivariate logistic regression analysis showed that the superior direction of aneurysm dome (OR 9.106, p = 0.007), the presence of a bleb (OR 4.780, p < 0.001), a high aspect ratio (OR 1.878, p = 0.045), and fPCA (2.101, p = 0.040) were significantly associated with PcomA aneurysm rupture. In the fPCA group, only the presence of a bleb varied significantly between ruptured and unruptured PcomA aneurysms. However, in the non-fPCA group, larger aneurysms, the superior direction of dome, the presence of a bleb, and a high aspect and dome-to-neck ratio were significantly higher in the ruptured aneurysm group than in the unruptured aneurysm group.
Conclusions
The results demonstrate that fPCA may be an independent risk factor for rupture, especially together with the presence of a bleb.

Similar content being viewed by others

References
Golshani K, Ferrell A, Zomorodi A, Smith TP, Britz GW (2010) A review of the management of posterior communicating artery aneurysms in the modern era. Surg Neurol Int 1:88. https://doi.org/10.4103/2152-7806.74147
Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366:2474–2482. https://doi.org/10.1056/NEJMoa1113260
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Brown RD Jr, Broderick JP (2014) Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol 13:393–404. https://doi.org/10.1016/s1474-4422(14)70015-8
Fogelholm R, Hernesniemi J, Vapalahti M (1993) Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage. A population-based study. Stroke 24:1649–1654
van Gijn J, Kerr RS, Rinkel GJ (2007) Subarachnoid haemorrhage. Lancet 369:306–318. https://doi.org/10.1016/s0140-6736(07)60153-6
Baharoglu MI, Lauric A, Gao BL, Malek AM (2012) Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J Neurosurg 116:871–881. https://doi.org/10.3171/2011.11.Jns11311
Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH, Reavey-Cantwell JF, Lewis SB (2007) Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery 61:716–722; discussion 722-713. https://doi.org/10.1227/01.Neu.0000298899.77097.Bf
Prestigiacomo CJ, He W, Catrambone J, Chung S, Kasper L, Pasupuleti L, Mittal N (2009) Predicting aneurysm rupture probabilities through the application of a computed tomography angiography-derived binary logistic regression model. J Neurosurg 110:1–6. https://doi.org/10.3171/2008.5.17558
Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, Nakajima H, Hori T, Takakura K, Kajiya F (1999) Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery 45:119–129 discussion 129-130
Frosen J (2016) Flow dynamics of aneurysm growth and rupture: challenges for the development of computational flow dynamics as a diagnostic tool to detect rupture-prone aneurysms. Acta Neurochir Suppl 123:89–95. https://doi.org/10.1007/978-3-319-29887-0_13
Hassan T, Timofeev EV, Saito T, Shimizu H, Ezura M, Matsumoto Y, Takayama K, Tominaga T, Takahashi A (2005) A proposed parent vessel geometry-based categorization of saccular intracranial aneurysms: computational flow dynamics analysis of the risk factors for lesion rupture. J Neurosurg 103:662–680. https://doi.org/10.3171/jns.2005.103.4.0662
Arjal RK, Zhu T, Zhou Y (2014) The study of fetal-type posterior cerebral circulation on multislice CT angiography and its influence on cerebral ischemic strokes. Clin Imaging 38:221–225. https://doi.org/10.1016/j.clinimag.2014.01.007
He Z, Wan Y (2018) Is fetal-type posterior cerebral artery a risk factor for intracranial aneurysm as analyzed by multislice CT angiography? Exp Ther Med 15:838–846. https://doi.org/10.3892/etm.2017.5504
Lv X, Li Y, Yang X, Jiang C, Wu Z (2012) Potential proneness of fetal-type posterior cerebral artery to vascular insufficiency in parent vessel occlusion of distal posterior cerebral artery aneurysms. J Neurosurg 117:284–287. https://doi.org/10.3171/2012.4.Jns111788
Lv N, Feng Z, Wang C, Cao W, Fang Y, Karmonik C, Liu J, Huang Q (2016) Morphological risk factors for rupture of small (<7 mm) posterior communicating artery aneurysms. World Neurosurg 87:311–315. https://doi.org/10.1016/j.wneu.2015.12.055
Matsukawa H, Fujii M, Akaike G, Uemura A, Takahashi O, Niimi Y, Shinoda M (2014) Morphological and clinical risk factors for posterior communicating artery aneurysm rupture. J Neurosurg 120:104–110. https://doi.org/10.3171/2013.9.Jns13921
Zhang Y, Jing L, Liu J, Li C, Fan J, Wang S, Li H, Yang X (2016) Clinical, morphological, and hemodynamic independent characteristic factors for rupture of posterior communicating artery aneurysms. J Neurointerv Surg 8:808–812. https://doi.org/10.1136/neurintsurg-2015-011865
Shaban A, Albright KC, Boehme AK, Martin-Schild S (2013) Circle of Willis variants: fetal PCA. Stroke Res Treat 2013:105937–105936. https://doi.org/10.1155/2013/105937
Xu J, Yu Y, Wu X, Wu Y, Jiang C, Wang S, Huang Q, Liu J (2013) Morphological and hemodynamic analysis of mirror posterior communicating artery aneurysms. PLoS One 8:e55413. https://doi.org/10.1371/journal.pone.0055413
Lin W, Ma X, Deng D, Li Y (2015) Hemodynamics in the circle of Willis with internal carotid artery stenosis under cervical rotatory manipulation: a finite element analysis. Med Sci Monit 21:1820–1826. https://doi.org/10.12659/msm.892822
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Z., Kim, B.S., Lee, K.S. et al. Morphological and clinical risk factors for the rupture of posterior communicating artery aneurysms: significance of fetal-type posterior cerebral artery. Neurol Sci 40, 2377–2382 (2019). https://doi.org/10.1007/s10072-019-03991-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03991-4