Abstract
Background
Superficial siderosis (SS) of the central nervous system is a rare and heterogeneous condition due to deposition of hemosiderin on the surface of the brain and spinal cord. The usually progressive clinical course is characterized by a combination of hearing loss, cerebellar ataxia, and myelopathy. There is no known treatment for SS, but the iron chelator deferiprone (DFP) has been proposed as a potentially useful treatment.
Methods
We present a long-term (average 3.7 years) evaluation of four cases of SS treated with DFP (15 mg/kg po bid).
Results
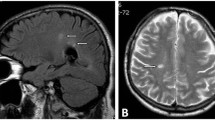
Treatment with DFP proved safe and well tolerated. Two out of the four subjects were unchanged while the other two presented a clinical improvement with reduction of postural instability and cerebellar signs. Blinded evaluation of magnetic resonance imaging (performed every 6 months during follow-up) showed a reduction of the abnormal iron deposition for all patients.
Conclusions
This long-term observational study suggests that DFP may be effective in the management of the neurological manifestations associated with iron accumulation in SS.
Clinicaltrials.gov identifier
NTC00907283
Similar content being viewed by others
References
Fearnley JM, Stevens JM, Rudge P (1995) SS of the central nervous system. Brain 118:1051–1066
Levy M, Turtzo C, Llinas RH (2007) Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neurol 3:54–58
Kumar N, Lane JI, Piepgras DG (2009) Superficial siderosis: sealing the defect. Neurology 72:671–673
Koeppen AH, Dentinger MP (1988) Brain hemosiderin and SS of the central nervous system. J Neuropathol Exp Neurol 47:249–270
Parnes SM, Weaver SA (1992) Superficial Siderosis of the central nervous system: a neglected cause of sensorineural hearing loss. Otolaryngol Head Neck Surg 107:69–77
Fredenburg AM, Sethi RK, Allen DD, Yokel RA (1996) The pharmacokinetics and blood-brain barrier permeation of the chelators 1,2 dimethyl-, 1,2 diethyl-,and 1-[ethan-1’ol]-2-methyl-3-hydroxypyridin-4-one in the rat. Toxicology 108:191e9
Abbruzzese G, Cossu G, Balocco M, Marchese R, Murgia D, Melis M, Galanello R, Barella S, Matta G, Ruffinengo U, Bonuccelli U, Forni GL (2011) A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica 96:1708–1711
Cossu G, Abbruzzese G, Matta G, Murgia D, Melis M, Ricchi V, Galanello R, Barella S, Origa R, Balocco M, Pelosin E, Marchese R, Ruffinengo U, Forni GL (2014) Efficacy and safety of deferiprone for the treatment of pantothenate kinase-associated neurodegeneration (PKAN) and neurodegeneration with brain iron accumulation (NBIA): results from a four years follow-up. Parkinsonism Relat Disord 20:651–654
Pandolfo M, Arpa J, Delatycki MB, Le Quan Sang KH, Mariotti C, Munnich A, Sanz-Gallego I, Tai G, Tarnopolsky MA, Taroni F, Spino M, Tricta F (2014) Deferiprone in Friedreich ataxia: a 6-month randomized controlled trial. Ann Neurol 76:509–521
Cummins G, Crundwell G, Baguley D, Lennox G (2013) Treatment of superficial siderosis with iron chelation therapy. BMJ Case Rep 2013:bcr2013009916. https://doi.org/10.1136/bcr-2013-009916
Levy M, Llinas R (2012) Update on a patient with superficial siderosis on deferiprone. AJNR Am J Neuroradiol 33:E99–E100
Levy M, Llinas R (2012) Pilot safety trial of deferiprone in 10 subjects with superficial siderosis. Stroke 43:120–124
Kessler RA, Li X, Schwartz K, Huang H, Mealy MA, Levy M (2018) Two-year observational study of deferiprone in superficial siderosis. CNS Neurosci Ther 24:187–192
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The trial was approved by the E.O. Ospedali Galliera Ethics Committee, and all participants gave written informed consent before entering the study (Clinicaltrials.gov identifier: NTC00907283).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cossu, G., Abbruzzese, G., Forni, G.L. et al. Efficacy and safety of deferiprone for the treatment of superficial siderosis: results from a long-term observational study. Neurol Sci 40, 1357–1361 (2019). https://doi.org/10.1007/s10072-019-03847-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03847-x