Abstract
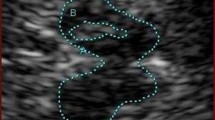
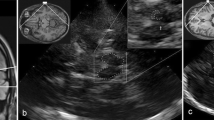
Transcranial sonography (TCS) is a noninvasive, easily performed, and commonly available neuroimaging technique useful for the study of brain parenchyma in movement disorders. This tool has been increasingly used in the diagnosis of Parkinson’s disease and atypical parkinsonism. The aim of the study was to evaluate the applicability of this technique as supportive tool in the early diagnosis of movement disorders. We performed TCS on 315 individuals which were diagnosed as healthy controls or affected by idiopathic Parkinson’s disease, monogenetic subtypes of Parkinson’s disease, atypical parkinsonism, and Dementia with Lewy bodies. Five TCS diagnostic patterns were defined on the basis of substantia nigra’s and lenticular nuclei’s echogenicity. TCS evaluations were performed by two blinded neuro-sonographers. Clinical diagnosis on all individuals was performed at baseline and at 4-year follow-up. The concordance rate between TCS patterns and clinical diagnosis and the specificity of TCS pattern to discriminate each group of individuals were compared at baseline and at follow-up. The concordance rate between TCS patterns and clinical diagnosis of all individuals was 84% at baseline and increased at follow-up (91%) significantly (p = 0.01). The specificity of TCS pattern in the comparison between patients diagnosed as affected by idiopathic Parkinson’s disease and atypical parkinsonism showed a significant increase at follow-up (p = 0.03). Our study strongly confirms the role of TCS as a noninvasive and cost-effective tool in early diagnosis of movement disorders.

Similar content being viewed by others
References
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576
Pilotto A, Yilmaz R, Berg D (2015) Developments in the role of transcranial sonography for the differential diagnosis of parkinsonism. Curr Neurol Neurosci Rep 15(7):43
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45:182–184
Walter U, Dressler D, Wolters A, Wittstock M, Greim B, Benecke R (2006) Sonographic discrimination of dementia with Lewy bodies and Parkinson’s disease with dementia. J Neurol 253(4):448–454
Schweitzer KJ, Brüssel T, Leitner P, Krüger R, Bauer P, Woitalla D, Tomiuk J, Gasser T, Berg D (2007) Transcranial ultrasound in different monogenetic subtypes of Parkinson’s disease. J Neurol 254(5):613–616
Venegas-Francke P (2010) Transcranial sonography in the discrimination of Parkinson’s disease versus vascular parkinsonism. Int Rev Neurobiol 90:147–156
Bouwmans AE, Vlaar AM, Srulijes K, Mess WH, Weber WE (2010) Transcranial sonography for the discrimination of idiopathic Parkinson’s disease from the atypical parkinsonian syndromes. Int Rev Neurobiol 90:121–146
Iranzo A, Lomeña F, Stockner H, Valldeoriola F, Vilaseca I, Salamero M, Molinuevo JL, Serradell M, Duch J, Pavía J, Gallego J, Seppi K, Högl B, Tolosa E, Poewe W, Santamaria J (2010) Sleep Innsbruck Barcelona (SINBAR) pattern. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behavior disorder: a prospective study. Lancet Neurol 9(11):1070–1077
Berg D, Seppi K, Behnke S, Liepelt I, Schweitzer K, Stockner H, Wollenweber F, Gaenslen A, Mahlknecht P, Spiegel J, Godau J, Huber H, Srulijes K, Kiechl S, Bentele M, Gasperi A, Schubert T, Hiry T, Probst M, Schneider V, Klenk J, Sawires M, Willeit J, Maetzler W, Fassbender K, Gasser T, Poewe W (2011) Enlarged substantia nigra hyperechogenicity and risk for Parkinson disease: a 37-month 3-center study of 1847 older persons. Arch Neurol 68(7):932–937
Berg D, Merz B, Reiners K, Naumann M, Becker G (2005) Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disord 20(3):383–385
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65:1863–1872
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Mathew R, Bak TH, Hodges JR (2012) Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry 83(4):405–410
Berg D, Godau J, Walter U (2008) Transcranial sonography in movement disorders. Lancet Neurol 7:1044–1055
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D (2003) Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 60:74–77
Walter U, Dressler D, Wolters A, Probst T, Grossmann A, Benecke R (2004) Sonographic discrimination of corticobasal degeneration vs progressive supranuclear palsy. Neurology 63:504–509
Walter U, Dressler D, Wolters A, Wittstock M, Benecke R (2007) Transcranial brain sonography findings in clinical subgroups of idiopathic Parkinson’s disease. Mov Disord 22:48–54
Bartova P, Skoloudik D, Bar M, Ressner P, Hlustik P, Herzig R, Kanovsky P (2008) Transcranial sonography in movement disorders. Biomed Pap Med FacUnivPalacky Olomouc Czech Repub 152(2):251–258
Gaenslen A, Unmuth B, Godau J, Liepelt I, Di Santo A, Johanna Schweitzer K, Gasser T, Machulla HJ, Reimold M, Marek K, Berg D (2008) The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson’s disease: a prospective blinded study. Lancet Neurol 7:417–424
Schrag A, Good CD, Miszkiel K, Morris HR, Mathias CJ, Lees AJ, Quinn NP (2000) Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 54(3):697–702
Dickson DW, Anderton B, Morris H et al (2001) International medical workshop covering progressive supranuclear palsy, multiple system atrophy and corticobasal degeneration. Mov Disord 16:382–395
Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, Brooks D, Burn DJ, Colosimo C, Fanciulli A, Ferreira J, Gasser T, Grandas F, Kanovsky P, Kostic V, Kulisevsky J, Oertel W, Poewe W, Reese JP, Relja M, Ruzicka E, Schrag A, Seppi K, Taba P, Vidailhet M (2013) EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. New European guidelines for the diagnosis of Parkinson’s disease. TCS evaluation has a level a of evidence in early and differential diagnosis of PD. Eur J Neurol 20:16–34
Li X, Xue S, Jia S, Zhou Z, Qiao Y, Hou C, Wei K, Zheng W, Rong P, Jiao J (2017) Transcranial sonography in idiopathic REM sleep behavior disorder and multiple system atrophy. Psychiatry Clin Neurosci 71(4):238–246
Sanzaro E, Iemolo F (2016) Transcranial sonography in movement disorders: an interesting tool for diagnostic perspectives. Neurol Sci 37(3):373–376
Li DH, He YC, Liu J, Chen SD (2016) Diagnostic accuracy of transcranial sonography of the substantia nigra in Parkinson’s disease: a systematic review and meta-analysis. Sci Rep 6:20863
Berg D, Becker G, Zeiler B, Tucha O, Hofmann E, Preier M, Benz P, Jost W, Reiners K, Lange KW (1999) Vulnerability of the nigrostriatal system as detected by transcranial ultrasound. Neurology 53:1026–1031
Berg D, Roggendorf W, Schroder U et al (2002) Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol 59:999–1005
Berg D, Behnke S, Seppi K, Godau J, Lerche S, Mahlknecht P, Liepelt-Scarfone I, Pausch C, Schneider N, Gaenslen A, Brockmann K, Srulijes K, Huber H, Wurster I, Stockner H, Kiechl S, Willeit J, Gasperi A, Fassbender K, Gasser T, Poewe W (2013) Enlarged hyperechogenic substantia nigra as a risk marker for Parkinson’s disease. Mov Disord 28(2):216–219
deRijk MC, Tzourio C, Breteler MM et al (1997) Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON collaborative study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:10–15
Zecca L, Swartz HM (1993) Total and paramagnetic metals in human substantia nigra and its neuromelanin. J Neural Transm Park Dis Dement 3:203–213
Berg D, Godau J, Riederer P, Gerlach M, Arzberger T (2010) Microglia activation is related to substantia nigra echogenicity. J Neural Transm 117:1287–1292
Li DH, Zhang LY, Hu YY, Jiang XF, Zhou HY, Yang Q, Kang WY, Liu J, Chen SD (2015) Transcranial sonography of the substantia nigra and its correlation with DAT-SPECT in the diagnosis of Parkinson’s disease. Parkinsonism Relat Disord 21:923–928
Fengler S, Liepelt-Scarfone I, Brockmann K, Schäffer E, Berg D, Kalbe E (2017) Cognitive changes in prodromal Parkinson’s disease: a review. Mov Disord 32(12):1655–1666
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of informed consent
The study was approved by the ethics committee. Written informed consent was obtained from each participant before the inclusion in the study.
Rights and permissions
About this article
Cite this article
Monaco, D., Berg, D., Thomas, A. et al. The predictive power of transcranial sonography in movement disorders: a longitudinal cohort study. Neurol Sci 39, 1887–1894 (2018). https://doi.org/10.1007/s10072-018-3514-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3514-z