Abstract
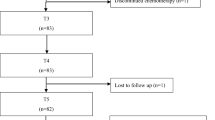
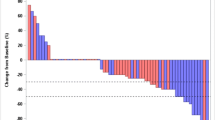
Many chemotherapy treatments induce peripheral neuropathy (CIPN). These patients often experience neuropathic pain (NP) that reduces the quality of life. The aim of this prospective, open label study was to evaluate the efficacy and tolerability of tapentadol (TP) in patients affected by CIPN. CIPN were consecutively enrolled in a prospective open label study at the Neuro-Oncology Unit of the Regina Elena National Cancer Institute in Rome. During the titration phase, each patient initially received doses of TP 50 mg twice a day. All patients underwent pain intensity (NRS) and DN4. For evaluation of quality of life, patients underwent EORTC QLQ-C30 and EORTC QLQ-CIPN2 QLQ-CIPN20. We enrolled 31 patients, 19 were females with a median age of 60 years. After 3 months of treatment with TP, 22 patients completed the statistical package for social sciences (SPSS). Nineteen patients out of 22 showed a response to treatment (86%). We also observed that TP reduced the NRS and DN4 values from baseline to the last visit in a significant way (p < 0.001, respectively). Seven patients (22.5%) discontinued the TP therapy after the first week of occurrence of side effects. Furthermore, we observed that TP improved also the global health status measured by EORT QLQ-C30. TP is well tolerated and efficacy in the treatment of NP. The important reduction of neuropathic pain, the improvement in NRS and QoL scores after therapy with TP makes it a candidate in the management of patients suffering from neuropathic pain of CIPN also as a first line of therapy.

Similar content being viewed by others
References
Piano V, Verhagen S, Schalkwijk A, Burgers J, Kress H, Treede RD, Hekster Y, Lanteri-Minet M, Engels Y, Vissers K (2013) Diagnosing neuropathic pain in patients with cancer: comparative analysis of recommendations in national guidelines from European countries. Pain Pract 13:433–439. doi:10.1111/papr.12018 Epub 2012 Dec 19
Delforge M, Bladé J, Dimopoulos MA, Facon T, Kropff M, Ludwig H, Palumbo A, Van Damme P, San-Miguel JF, Sonneveld P (2010) Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol 11:1086–1095. doi:10.1016/S1470-2045(10)70068-1
Windebank AJ, Grisold W (2008) Chemotherapy-induced neuropathy. J Peripheral Nerv Syst 13:27–46. doi:10.1111/j.1529-8027.2008.00156.x
Cavaletti G, Zanna C (2002) Current status and future prospects for the treatment of chemotherapy-induced peripheral neurotoxicity. Eur J Cancer 38:1832–1837
Rayment C, Hjermstad MJ, Aass N, Kaasa S, Caraceni A, Strasser F, Heitzer E, Fainsinger R, Bennett MI (2013) Neuropathic cancer pain: prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative—computerised symptom assessment study. Palliat Med 27:714–721. doi:10.1177/0269216312464408 Epub 2012 Nov 21
Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP (2008) Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 66:218–228. doi:10.1016/j.critrevonc.2008.01.008 Epub 2008 Mar 7
Cavaletti G Peripheral neurotoxicity of platinum-based chemotherapy. Nat Rev Cancer 8, 2008. Doi: 10.1038/nrc2167-c1
Cassier PA, Walter T, Eymard B, Ardisson P, Perol M, Paillet C, Chayvialle JA, Scoazec JY, Hervieu V, Bohas CL (2009) Gemcitabine and oxaliplatin combination chemotherapy for metastatic well-differentiated neuroendocrine carcinomas: a single-center experience. Cancer 115:3392–3399. doi:10.1002/cncr.24384
Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S (2012) Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain 153:359–365. doi:10.1016/j.pain.2011.10.028 Epub 2011 Nov 23
Merskey H, Bogduk N (1994) Part III: pain terms, a current list with definitions and notes on usage. In: Classification of chronic pain, IASP task force on taxonomy, 2nd edn. IASP Press, Seattle, pp 209–214
Baron R, Binder A, Wasner G (2010) Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 9:807–819. doi:10.1016/S1474-4422(10)70143-5
Zhuo M, Wu G, Wu LJ (2011) Neuronal and microglial mechanisms of neuropathic pain. Mol Brain 4:1.51. doi:10.1186/1756-6606-4-31
Beijers AJ, Jongen JL, Vreugdenhil G (2012) Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies. Neth J Med 70:18–25
Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, Seisler D, Qamar R, Lewis GC, Grothey A (2014) Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol 32:997–1005. doi:10.1200/JCO.2013.52.0536 Epub 2013 Dec 2
Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T (2010) EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European Journal of Neurology 17:1113–1123. doi:10.1111/j.1468-1331.2010.02999.x Epub 2010 Apr 9
Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, Kent JL, Krane EJ, Lebel AA, Levy RM, Mackey SC, Mayer J, Miaskowski C, Raja SN, Rice AS, Schmader KE, StaceyB SS, Treede RD, Turk DC, Walco GA, Wells CD (2010) Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc 85(3 Suppl):S3–14
Park HJ, Moon DE (2010) Pharmacologic Management of Chronic Pain. Korean J Pain 23:99–108. doi:10.3344/kjp.2010.23.2.99 Epub 2010 May 31
Coluzzi F, Raffa RB, Pergolizzi J, Rocco A, Locarini P, Cenfra N, Cimino G, Mattia C (2015) Tapentadol prolonged release for patients with multiple myeloma suffering from moderate-to-severe cancer pain due to bone disease. J Pain Res 8:229–238. doi:10.2147/JPR.S83490
Mercadante S, Porzio G, Ferrera P, Aielli F, Adile C, Ficorella C, Giarratano A, Casuccio A (2012) Curr Med Res Opin 28:1775–1779. doi:10.1185/03007995.2012.739151
Mercadante S, Porzio G, Adile C, Aielli F, Cortegiani A, Dickenson A, Casuccio A (2014) Tapentadol at medium to high doses in patients previously receiving strong opioids for the management of cancer pain. Curr Med Res Opin 30:2063–2068. doi:10.1185/03007995.2014.934793
Pergolizzi J, Alon E, Baron R, Bonezzi C, Dobrogowski J, Gálvez R, Jensen T, Kress HG, Marcus MA, Morlion B, Perrot S, Treede RD (2011) Tapentadol in the management of chronic low back pain: a novel approach to a complex condition. J Pain Res 203:210. doi:10.2147/JPR.S19625
Pierce DM, Shipstone E (2012) Pharmacology update: tapentadol for neurophatic pain. Am J Hosp Palliat Care 29:663–666. doi:10.1177/1049909111434634
Prommer EE (2010) Tapentadol: an initial analysis. J Opioid Manag J Opioid Manag 6:223–226
Riemsma R, Forbes C, Harker J, Worthy G, Misso K, Schäfer M, Kleijnen J, Stürzebecher S (2011) Systemic review of tapentadol in chronic severe pain. Curr Med Res Opin 27:1907–1930. doi:10.1185/03007995.2011.611494
Schikowski A, Krings D, Schwenke K (2014) Tapentadol prolonged release for severe chronic cancer-related pain: effectiveness, tolerability, and influence on quality of life of the patients. J Pain Res 8:1–8. doi:10.2147/JPR.S72150
Steigerwald I, Müller M, Davies A, Samper D, Sabatowski R, Baron R, Rozenberg S, Szczepanska-Szerej A, Gatti A, Kress HG (2012) Effectiveness and safety of tapentadol prolonged release for severe, chronic low back pain with or without a neuropathic pain component: results of open-label, phase 3b study. Curr Med Res Opin 28:911–936. doi:10.1185/03007995.2012.679254
Vadivelu N, Kai A, Maslin B, Kodumudi G, Legler A, Berger JM (2015) Tapentadol extended release in the management of peripheral diabetic neuropathic pain. Ther Clin Risk Manag 11:95–105. doi:10.2147/TCRM.S32193
Vadivelu N, Huang Y, Mirante B, Jacoby M, Braveman FR, Hines RL, Sinatra R (2013) Patient considerations in the use of tapentadol for moderate to severe pain. Drug Healthc Patient Saf 5:151–159. doi:10.2147/DHPS.S28829
Vadivelu N, Timchenko A, Huang Y, Sinatra R (2011) Tapentadol extended-release for treatment of chronic pain: a review. J Pain Res 4:211–218. doi:10.2147/JPR.S14842
Kress HG (2010) Tapentadol and its two mechanisms of action: is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur J Pain 14:781–783. doi:10.1016/j.ejpain.2010.06.017
Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19. doi:10.1016/j.pain.2004.09.012
Van Seventer R, Vos C, Meerding W, Mear I, Le Gal M, Bouhassira D, Huygen FJ (2010) Linguistic validation of the DN4 for use in international studies. Eur J Pain 14:58–63. doi:10.1016/j.ejpain.2009.01.005
Pérez C, Sánchez-Martínez N, Ballesteros A, Blanco T, Collazo A, González F, Villoria J (2015) Prevalence of pain and relative diagnostic performance of screening tools for neuropathic pain in cancer patients: a cross-sectional study. Eur J Pain 19:752–761. doi:10.1002/ejp.598
Giesinger JM, Kieffer JM, Fayers PM, Groenvold M, Petersen MA, Scott NW, Sprangers MA, Velikova G, Aaronson NK (2016) Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol 69:79–88. doi:10.1016/j.jclinepi.2015.08.007
Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL (2013) Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res 22:2787–2799. doi:10.1007/s11136-013-0379-8
Coluzzi F, Ruggeri M (2014) Clinical and economic evaluation of tapentadol extended release and oxycodone/naloxone extended release in comparison with controlled release oxycodone in musculoskeletal pain. Curr Med Res Opin 30:1139–1151. doi:10.1185/03007995.2014.894501
Mohty B, El-Cheikh J, Yakoub-Agha I, Moreau P, Harousseau JL, Mohty M (2010) Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica 95:311–319. doi:10.3324/haematol.2009.012674
Tzschentke TM, Folgering JH, Flik G, De Vry J (2012) Tapentadol increases levels of noradrenaline in the rat spinal cord as measured byin vivo microdialysis. Neurosci Lett 24(507):151–155. doi:10.1016/j.neulet.2011.12.008
Kögel B, De Vry J, Tzschentke TM, Christoph T (2011) The antinociceptive and antihyperalgesic effect of tapentadol is partially retained in OPRM1 (μ-opioid receptor)knockout mice. Neurosci Lett 17(491):104–107. doi:10.1016/j.neulet.2011.01.014
Meneghini V, Cuccurazzu B, Bortolotto V, Ramazzotti V, Ubezio F, Tzschentke TM, Canonico PL, Grilli M (2014) The noradrenergic component in tapentadol action counteracts μ-opioid receptor-mediated adverse effects on adult neurogenesis. Mol Pharmacol 85:658–670. doi:10.1124/mol.113.091520
Acknowledgments
We thank Dr. Tania Merlino for her English language editing of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galiè, E., Villani, V., Terrenato, I. et al. Tapentadol in neuropathic pain cancer patients: a prospective open label study. Neurol Sci 38, 1747–1752 (2017). https://doi.org/10.1007/s10072-017-3035-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3035-1