Abstract
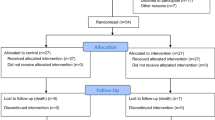
The only available treatment of traumatic spinal cord injury (TSCI) is high-dose methylprednisolone (MP) administered acutely after injury. However, as the efficacy of MP is controversial, we assessed the superiority of erythropoietin (EPO) versus MP in improving clinical outcome of acute TSCI. Patients aged 18 to 65 years after C5–T12 injury, and grade A or B of the ASIA Impairment Scale (AIS), admitted within 8 h, hemodynamically stable, were randomized to MP according to the NASCIS III protocol or EPO iv (500 UI/kg, repeated at 24 and 48 h). Patients were assessed by an investigator blind to treatment assignment at baseline and at day 3, 7, 14, 30, 60 and 90. Primary end point: number of responders (reduction of at least one AIS grade). Secondary end points: treatment safety and the effects of drugs on a number of disability measures. Frequentistic and post hoc Bayesian analyses were performed. Eight patients were randomized to MP and 11 to EPO. Three patients (27.3 %) on EPO and no patients on MP reached the primary end point (p = 0.17). No significant differences were found for the other disability measures. No adverse events or serious adverse events were reported in both groups. The Bayesian analysis detected a 91.8 % chance of achieving higher success rates on the primary end point with EPO in the intention-to-treat population with a 95 % chance the difference between EPO and MP falling in the range (−0.10, 0.51) and a median value of 0.2. The results of Bayesian analysis favored the experimental treatment.




Similar content being viewed by others
References
Schwab ME (2002) Repairing the injured spinal cord. Science 295:1029–1031
Kakulas BA (2004) Neuropathology: the foundation for new treatments in spinal cord injuries. Spinal Cord 42:549–563
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155
Gorio A, Madaschi L, Di Stefano B et al (2005) Methylprednisolone neutralizes the beneficial effects of erythropoietin in experimental spinal cord injury. Proc Natl Acad Sci USA 102:16379–16384
Bottai D, Cigognini D, Madaschi L et al (2010) Embryonic stem cells promote motor recovery and affect inflammatory cell infiltration in spinal cord injured mice. Exp Neurol 223:452–463
Kakulas BA (1999) The applied neuropathology of human spinal cord injury. Spinal Cord 37:79–88
Windle WF, Clemente CD, Scott D Jr, Chambers WW (1952) Structural and functional regeneration in the central nervous system. AMA Arch Neurol Psychiatry 67:553–554
Fawcett J (2002) Repair of spinal cord injuries: where we are, where are we going? Spinal Cord 40:615–623
Pointillart V, Petitjean M, Wiart J, Lassie P, Thicoipe M, Dabadie P (2000) Pharmacological therapy of spinal cord injury during the acute fase. Spinal Cord 38:71–76
Coleman WP, Benzel E, Cahill D et al (2000) A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in Acute Spinal Cord Injury. J Spinal Cord 13:185–199
Bracken MB (2000) Pharmacological interventions for acute spinal cord injury. Cochrane Database Syst Rev 2000(2):CD001046
Hall ED, Braughler JM (1982) Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg Neurol 18:320–327
Gorio A, Gokman N, Erbayraktar S et al (2002) Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Poc Natl Acad Sci USA 99:9450–9455
Vitellaro L, Mazzetti S, Maraschi L, Bosisio P, Gorio A, DeBiasi S (2007) Erythropoietin mediated preservation of the white matter in the spinal cord after traumatic injury. Neuroscience 144:865–877
Brines M, Cerami A (2006) Erythropoietin in spinal cord injury. Erythropoietin and the nervous system. Novel therapeutic options for neuroprotection. Springer, New York, pp 147–164
Bernaudin M, Marti HH, Roussel S et al (1999) A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab 19:643–651
Ehrenreich H, Weissenborn K, Prange H et al (2009) Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40:e647–e656
Wang L, Chopp M, Gregg SR et al (2008) Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab 28:1361–1368
Giese AK, Frahm J, Hübner R et al (2010) Erythropoietin and the effect of oxygen during proliferation and differentiation of human neural progenitor cells. BMC Cell Biol 11:94
Digicayloglu M, Lipton SA (2001) Erythropoietin mediated neuroprotection involves cross talk between jak-2 and NF-kB signaling 6 cascades. Nature 412:641–647
Lammertse D, Tuszynski MH, Steeves JD et al (2007) Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 45:232–242
American Spinal Injury Association, International Medical Society of Paraplegia (2002) International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association, Chicago (Revised 2002)
Bracken MB, Shepard MJ, Holford TR et al (1997) Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. JAMA 277:1597–1604
Catz A, Hzkovich M, Agranov E, Ring H, Tamir A (1997) SCIM- Spinal Cord Independence Measure: a new disability scale for patients with spinal cord lesions. Spinal Cord 35:850–856
Bohannon RW, Smith MB (1987) Interrater reliability of modified Ashworth scale of muscle spasticity. Phys Ther 67:206–207
Penn RD, Savoy SM, Corcos D et al (1989) Intratecal baclofen for severe spinal spasticity. N Engl J Med 320:155–214
Wewers ME, Lowe NK (1990) A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 13:227–236
Marino RJ, Ditunno JF Jr, Donovan WH, Maynard F Jr (1999) Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil 80:1391–1396
Waters RL, Yakura JS, Adkins RH, Sie I (1992) Recovery following complete paraplegia. Arch Phys Med Rehabil 73:784–789
O’Brien PC, Fleming TR (1979) A multiple testing procedure for clinical trials. Biometrics 35:549–556
Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H (2010) Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol 24:573–594
My Tseng, Hutchinson PJ, Richards HK et al (2009) Acute systemic erythropoietin therapy to reduce delayed ischemic deficits following aneurysmal subarachnoid hemorrhage: a phase II randomized, double blind, placebo-controlled trial. J Neurosurg 111:171–180
Carelli S, Marfia G, Di Giulio AM, Ghilardi G, Gorio A (2011) Erythropoietin: recent developments in the treatment of spinal cord injury. Neurol Res Int 453179
Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS (1997) Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 3:73–76
Totoiu MO, Keirstead HS (2005) Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 486:373–383
Cerri G, Montagna M, Madaschi L et al (2012) Erythropoietin effect on sensorimotor recovery after contusive spinal cord injury: an electrophysiological study. Neuroscience 219:290–301
Rossignol S, Giroux N, Chau C, Marcoux J, Brustein E, Reader TA (2001) Pharmacological Aids to locomotor training after spinal cord injury in the cat. J Physiol 533:65–74
Fawcett JW, Curt A, Steeves JD et al (2007) Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45:190–205
Steeves JD, Lammertse D, Curt A et al (2007) Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 45:206–221
Furlan JC, Noonan V, Singh A, Fehlings MG (2011) Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma 28:1445–1477
Acknowledgments
The study was funded by the Italian Drug Agency (Agenzia Italiana del Farmaco), contract number FARM6Y35XMI.
Conflict of interest
Ettore Beghi has received personal fees for board membership by VIROPHARMA and GSK; has received funding for travel and speaker honoraria from UCB-Pharma and GSK, for educational presentations from GSK; has received Grants for research activities from the Italian Drug Agency, Italian Ministry of Health, EISAI and the American ALS Association. Elisabetta Pupillo has received funding from the American ALS Association and Italian Ministry of Health for data management and data monitoring of an observational study protocol. She is receiving funding from Italian Drug Agency (AIFA) for data monitoring and study management of randomized clinical trial. Paolo Messina has received funding from Sanofi-Aventis, EISAI, Lombardy Region, and the American ALS Association for the data analysis and data management of RCT and observational study protocol. Paola Carignano, Davide Dalla Costa, Franco Faccioli, Alfredo Gorio, Cristina Pagliacci and Tiziana Redaelli: nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, D.D., Beghi, E., Carignano, P. et al. Tolerability and efficacy of erythropoietin (EPO) treatment in traumatic spinal cord injury: a preliminary randomized comparative trial vs. methylprednisolone (MP). Neurol Sci 36, 1567–1574 (2015). https://doi.org/10.1007/s10072-015-2182-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2182-5