Abstract
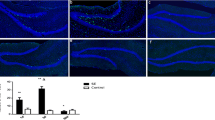
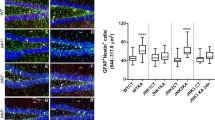
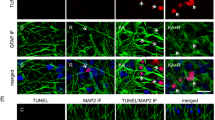
Hyperactivation of mammalian target of rapamycin (mTOR) signaling pathway occurs after an epileptogenic insult and, its inhibition prevents the development of spontaneous seizures. We have recently demonstrated that mTOR’s inhibition by rapamycin (started before seizure onset), permanently reduces the development of spontaneous absence seizures in WAG/Rij rats, an animal model of absence epilepsy; furthermore, mTOR phosphorylation was increased in adult WAG/Rij rats’ cortex, but not other brain areas. However, it was not clear whether this hyperphosphorylation was a cause or a consequence of absence seizure. Here, we have addressed this issue by analyzing immunohistochemically: (1) the brain levels of total and phosphorylated mTOR in young (before seizures) and adult WAG/Rij rats; (2) the proliferation of hippocampal neuronal stem/progenitor cells assessed by BrdU analysis at different ages. WAG/Rij rats have higher levels of total mTOR in several brain areas than Wistar rats; phospho-mTOR staining is higher in young WAG/Rij rats than control and adult WAG/Rij rats. Finally, the age-related decline in hippocampal neural progenitor cell proliferation rate was slower in WAG/Rij than Wistar rats. Our results support a role for persistent mTOR activation and consequent change in hippocampal progenitor cell proliferation during the epileptogenic process leading to the development of absence seizures in WAG/Rij rats.



Similar content being viewed by others
References
Russo E, Citraro R, Constanti A, De Sarro G (2012) The mTOR signaling pathway in the brain: focus on epilepsy and epileptogenesis. Mol Neurobiol 46(3):662–681. doi:10.1007/s12035-012-8314-5
Waltereit R, Welzl H, Dichgans J, Lipp HP, Schmidt WJ, Weller M (2006) Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem 96(2):407–413. doi:10.1111/j.1471-4159.2005.03538.x
Sosunov AA, Wu X, McGovern RA, Coughlin DG, Mikell CB, Goodman RR, McKhann GM 2nd (2012) The mTOR pathway is activated in glial cells in mesial temporal sclerosis. Epilepsia 53(Suppl 1):78–86. doi:10.1111/j.1528-1167.2012.03478.x
van Luijtelaar G, Zobeiri M (2014) Progress and outlooks in a genetic absence epilepsy model (WAG/Rij). Curr Med Chem 21(6):704–721
Russo E, Citraro R, Scicchitano F, De Fazio S, Perrotta I, Di Paola ED, Constanti A, De Sarro G (2011) Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia 52(7):1341–1350. doi:10.1111/j.1528-1167.2011.03112.x
Russo E, Citraro R, Scicchitano F, Urzino A, Marra R, Rispoli V, De Sarro G (2011) Vigabatrin has antiepileptogenic and antidepressant effects in an animal model of epilepsy and depression comorbidity. Behav Brain Res 225(1):373–376. doi:10.1016/j.bbr.2011.07.030
Russo E, Citraro R, Donato G, Camastra C, Iuliano R, Cuzzocrea S, Constanti A, De Sarro G (2013) mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology 69:25–36. doi:10.1016/j.neuropharm.2012.09.019
Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, De Sarro G (2010) Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia 51(8):1560–1569. doi:10.1111/j.1528-1167.2009.02400.x
Sandsmark DK, Pelletier C, Weber JD, Gutmann DH (2007) Mammalian target of rapamycin: master regulator of cell growth in the nervous system. Histol Histopathol 22(8):895–903
Paliouras GN, Hamilton LK, Aumont A, Joppe SE, Barnabe-Heider F, Fernandes KJ (2012) Mammalian target of rapamycin signaling is a key regulator of the transit-amplifying progenitor pool in the adult and aging forebrain. J Neurosci 32(43):15012–15026. doi:10.1523/JNEUROSCI.2248-12.2012
Pitkanen A, Lukasiuk K (2011) Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 10(2):173–186. doi:10.1016/S1474-4422(10)70310-0
Citraro R, Scicchitano F, De Fazio S, Raggio R, Mainardi P, Perucca E, De Sarro G, Russo E (2011) Preclinical activity profile of alpha-lactalbumin, a whey protein rich in tryptophan, in rodent models of seizures and epilepsy. Epilepsy Res 95(1–2):60–69. doi:10.1016/j.eplepsyres.2011.02.013
Citraro R, Russo E, Scicchitano F, van Rijn CM, Cosco D, Avagliano C, Russo R, D’Agostino G, Petrosino S, Guida F, Gatta L, van Luijtelaar G, Maione S, Di Marzo V, Calignano A, De Sarro G (2013) Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-alpha receptor activation in a genetic model of absence epilepsy. Neuropharmacology 69:115–126. doi:10.1016/j.neuropharm.2012.11.017
Russo E, Scicchitano F, Citraro R, Aiello R, Camastra C, Mainardi P, Chimirri S, Perucca E, Donato G, De Sarro G (2012) Protective activity of alpha-lactalbumin (ALAC), a whey protein rich in tryptophan, in rodent models of epileptogenesis. Neuroscience 226:282–288. doi:10.1016/j.neuroscience.2012.09.021
Citraro R, Russo E, Gratteri S, Di Paola ED, Ibbadu GF, Curinga C, Gitto R, Chimirri A, Donato G, De Sarro G (2006) Effects of non-competitive AMPA receptor antagonists injected into some brain areas of WAG/Rij rats, an animal model of generalized absence epilepsy. Neuropharmacology 51(6):1058–1067. doi:10.1016/j.neuropharm.2006.06.014
Macias M, Blazejczyk M, Kazmierska P, Caban B, Skalecka A, Tarkowski B, Rodo A, Konopacki J, Jaworski J (2013) Spatiotemporal characterization of mTOR kinase activity following kainic acid induced status epilepticus and analysis of rat brain response to chronic rapamycin treatment. PLoS ONE 8(5):e64455. doi:10.1371/journal.pone.0064455
Copp J, Manning G, Hunter T (2009) TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res 69(5):1821–1827. doi:10.1158/0008-5472.CAN-08-3014
Palmer TD, Takahashi J, Gage FH (1997) The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci 8(6):389–404. doi:10.1006/mcne.1996.0595
Biggio F, Gorini G, Utzeri C, Olla P, Marrosu F, Mocchetti I, Follesa P (2009) Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Int J Neuropsychopharmacol 12(9):1209–1221. doi:10.1017/S1461145709000200
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4(11):1313–1317
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16(6):2027–2033
Coggeshall RE, Lekan HA (1996) Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol 364(1):6–15
Russo E, Citraro R, Davoli A, Gallelli L, Di Paola ED, De Sarro G (2013) Ameliorating effects of aripiprazole on cognitive functions and depressive-like behavior in a genetic rat model of absence epilepsy and mild-depression comorbidity. Neuropharmacology 64:371–379. doi:10.1016/j.neuropharm.2012.06.039
Citraro R, Russo E, Ngomba RT, Nicoletti F, Scicchitano F, Whalley BJ, Calignano A, De Sarro G (2013) CB1 agonists, locally applied to the cortico-thalamic circuit of rats with genetic absence epilepsy, reduce epileptic manifestations. Epilepsy Res 106(1–2):74–82. doi:10.1016/j.eplepsyres.2013.06.004
Chen L, Hu L, Dong JY, Ye Q, Hua N, Wong M, Zeng LH (2012) Rapamycin has paradoxical effects on S6 phosphorylation in rats with and without seizures. Epilepsia 53(11):2026–2033. doi:10.1111/epi.12013
Shetty AK, Hattiangady B, Shetty GA (2005) Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia 51(3):173–186. doi:10.1002/glia.20187
Onat FY, van Luijtelaar G, Nehlig A, Snead OC 3rd (2013) The involvement of limbic structures in typical and atypical absence epilepsy. Epilepsy Res 103(2–3):111–123. doi:10.1016/j.eplepsyres.2012.08.008
Ates N, Esen N, Ilbay G (1999) Absence epilepsy and regional blood-brain barrier permeability: the effects of pentylenetetrazole-induced convulsions. Pharmacol Res 39(4):305–310. doi:10.1006/phrs.1998.0441
Zhang B, Wong M (2012) Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia 53(3):506–511. doi:10.1111/j.1528-1167.2011.03384.x
Conflict of interest
None of the authors has any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russo, E., Follesa, P., Citraro, R. et al. The mTOR signaling pathway and neuronal stem/progenitor cell proliferation in the hippocampus are altered during the development of absence epilepsy in a genetic animal model. Neurol Sci 35, 1793–1799 (2014). https://doi.org/10.1007/s10072-014-1842-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1842-1