Abstract
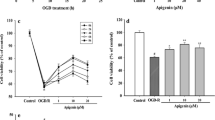
Our previous study showed that cinepazide maleate (CM) was as effective and safe as mildronate in the treatment of acute ischemic stroke in a randomized, double-blind, active-controlled phase II multicenter trial, but underlying mechanism(s) is not well understood. As an extending study, here we demonstrated that CM could protect neuronal cells by affecting mitochondrial functions. PC12 cells were exposed to 2.5 h oxygen–glucose deprivation (OGD) followed by a 24 h reoxygenation, and then treated with different concentrations (1, 10, 100 μM) of CM. Among various concentrations, 10 μM CM exhibited most significant protection on PC12 cells against OGD injury. CM was found to suppress OGD-induced oxidative stress, as supported by its capability of reducing intracellular reactive oxygen species and malondialdehyde production and enhancing superoxide dismutase activity. Importantly, our results showed that CM could preserve mitochondrial functions, as revealed by its capability of stabilizing mitochondrial membrane potential, improving OGD-induced suppression of mitochondrial respiratory complex activities and enhancing ATP production. In summary, our present study provides the first evidence that CM can protect neuronal cells against OGD injury by preserving mitochondrial functions.




Similar content being viewed by others
References
Hirohashi M, Hagihara Y (1979) Effect of 1-[(1-pyrrolidynylcarbonyl) methyl]-4-(3, 4, 5-trimethoxycinnamoyl)piperazine maleate (cinepazide) on cerebral and peripheral circulation in cats (author’s transl). Nihon Yakurigaku Zasshi 75(5):495–506
Moritoki H, Takei M, Fujita S, Ishida Y, Akashi A (1980) Interaction of cinepazide with adenosine on guinea-pig atria. Arch Int Pharmacodyn Ther 248(2):212–224
Muramatsu I, Sakakibara Y, Hong SC, Fujiwara M (1984) Effects of cinepazide on the purinergic responses in the dog cerebral artery. Pharmacology 28(1):27–33
Zhu Y, Zhang G, Zhao J, Li D, Yan X, Liu J, Liu X, Zhao H, Xia J, Zhang X, Li Z, Zhang B, Guo Z, Feng L, Zhang Z, Qu F, Zhao G (2013) Efficacy and safety of mildronate for acute ischemic stroke: a randomized, double-blind, active-controlled phase II multicenter trial. Clin Drug Investig 33(10):755–760
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67(2):181–198
Serteser M, Ozben T, Gumuslu S, Balkan S, Balkan E (2002) The effects of NMDA receptor antagonist MK-801 on lipid peroxidation during focal cerebral ischemia in rats. Prog Neuropsychopharmacol Biol Psychiatry 26(5):871–877
Xu L, Sun J, Lu R, Ji Q, Xu JG (2005) Effect of glutamate on inflammatory responses of intestine and brain after focal cerebral ischemia. World J Gastroenterol 11(5):733–736
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14(8):1505–1517
Gundimeda U, McNeill TH, Elhiani AA, Schiffman JE, Hinton DR, Gopalakrishna R (2012) Green tea polyphenols precondition against cell death induced by oxygen–glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cepsilon. J Biol Chem 287(41):34694–34708
Larsen EC, Hatcher JF, Adibhatla RM (2007) Effect of tricyclodecan-9-yl potassium xanthate (D609) on phospholipid metabolism and cell death during oxygen–glucose deprivation in PC12 cells. Neuroscience 146(3):946–961
Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, Sommer C, Schwab S (2003) Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 34(3):745–751
Cen J, Liu L, He L, Liu M, Wang CJ, Ji BS (2012) N(1)-(quinolin-2-ylmethyl)butane-1,4-diamine, a polyamine analogue, attenuated injury in in vitro and in vivo models of cerebral ischemia. Int J Dev Neurosci 30(7):584–595
Zhao Y, Wang B, Gao Y, Xiao Z, Zhao W, Chen B, Wang X, Dai J (2007) Olfactory ensheathing cell apoptosis induced by hypoxia and serum deprivation. Neurosci Lett 421(3):197–202
Rodrigo R, Fernandez-Gajardo R, Gutierrez R, Matamala JM, Carrasco R, Miranda-Merchak A, Feuerhake W (2013) Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol Disord Drug Targets 12(5):698–714
Zhang N, Komine-Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T (2005) Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 36(10):2220–2225
Warner DS, Sheng H, Batinic-Haberle I (2004) Oxidants, antioxidants and the ischemic brain. J Exp Biol 207(Pt 18):3221–3231
Iijima T (2006) Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res 55(3):234–243
Ly JD, Grubb DR, Lawen A (2003) The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 8(2):115–128
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344
Moro MA, Almeida A, Bolanos JP, Lizasoain I (2005) Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med 39(10):1291–1304
Drose S, Brandt U (2012) Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 748:145–169
Acknowledgments
We thank Ms. Dongyun Feng and Ms. Rui Wu for the technique support. This work was supported by the National Natural Science Foundation of China (Nos. 31170801 and 81371365) and by Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1053).
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Zhao and Y. Bai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, J., Bai, Y., Zhang, C. et al. Cinepazide maleate protects PC12 cells against oxygen–glucose deprivation-induced injury. Neurol Sci 35, 875–881 (2014). https://doi.org/10.1007/s10072-013-1618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-013-1618-z