Abstract
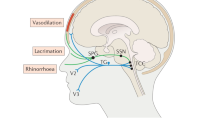
There are many categories and individual types of headache and most have a variety of treatment protocols, while a few are best treated by just one medication. This paper will concentrate on acute care medications for migraine and discuss some new and future acute care treatments. There is not much to discuss about prevention, except that onabotulinumtoxinA has been approved for prevention of chronic migraine. Cluster headache will also be discussed, as there are some future treatments for acute care and prevention being studied at present. For the acute care of migraine in the US, we have seven triptans by tablet plus other routes and one non steroidal anti-inflammatory medication approved by the FDA that is currently available (Cambia brand of buffered diclofenac potassium for oral solution). There are several other acute care medications in various stages of development and there are three new methods of administering a triptan and others under investigation. The optimal acute care therapy for migraine should be faster, easier to use and more efficient with fewer adverse events than what is currently available. What follows is a brief review of the status in development for five of the many new acute care medications being investigated: the CGRP antagonist tablet telcagepant, the sumatriptan iontophoretic patch Zelrix, sumatriptan powder for use in the OptiNose apparatus, dihydroergotamine for oral inhalation (Levadex), civamide nasal solution for prevention of episodic cluster headache (Civanex) and sphenopalatine ganglion stimulation for acute cluster attacks in chronic cluster headaches. Other future treatments that will not be discussed include transcranial magnetic stimulation, a 5-HT1F agonist named alniditan, large conductance calcium-activated potassium channel openers, glial modulators or other medications and devices in early stages of development.
Similar content being viewed by others
References
Ramadan NM, Buchanan TM (2006) New and future migraine therapy. Pharmacol Ther 112:199–212
Goadsby PJ, Edvinsson L (1993) The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuro- peptide changes seen in humans and cats. Ann Neurol 33:48–56
Levy D, Burstein R, Strassman AM (2005) Calcitonin gene related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol 58:698–705
Olesen J, Diener HC, Husstedt IW et al (2004) Calcitonin gene related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350:1104–1110
Ho TW, Mannix LK, Fan X et al (2008) Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 70:1304–1312
Ho TW, Ferrari MD, Dodick DW et al (2008) Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmi- triptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 372:2089–2090
Pierce M, Marbury T, O’Neill C et al (2008) A novel patch formulation of sumatriptan succinate utilizing SmartReliefTM transdermal technology. In: Data presented at the 50th annual scientific meeting of the American Headache Society, 28 June 2008
Acute Anti-Migraine Efficacy and Tolerability of Zelrix™, a Novel Iontophoretic Transdermal Patch of Sumatriptan. Paper presented at the American Academy of Neurology (AAN) 62nd Annual Meeting, April 2010
Rapoport AM, Freitag F, Pearlman S (2010) Innovative delivery systems for migraine: the clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs 24(11):929–940
Armer T, Shrewsbury S, Newman S et al (2007) Aerosol delivery of ergotamine tartrate via a breath-synchronized plume-control inhaler in humans. Curr Med Res Opin 23:3177–3187
Shrewsbury S, Cook R, Taylor G et al (2008) Safety and pharmacokinetics of dihydroergotamine mesylate administered via a novel (TEMPO) inhaler. Headache 48:355–367
Silberstein SD, Kori SH, Aurora S et al (2010) LEVADEX™, a novel orally inhaled treatment for acute migraine: Efficacy and tolerability results of a phase 3 study. In: 14th Congress of the International Headache Society; 2009 September 10–13, 2009; Philadelphia, PA, 2009
Luthringer R, Djupesland PG, Sheldrake CD et al (2009) Rapid absorption of sumatriptan powder and effects on the glyceryl trinitrate model of headache following intranasal delivery using a novel bi-directional device. J Pharm Pharmacol 61:1–10. doi:10.1211ISSN0022-3573
Djupesland PG, Docˇekal P, the Czech Migraine Investigators Group (2010) Intranasal sumatriptan powder delivered by a novel breath-actuated bi-directional device for the acute treatment of migraine: a randomised, placebo-controlled study. Cephalalgia 30(8):933–942 (Cephalalgia online first, published on March 17, 2010 as doi:10.1177/0333102409359314
Saper J, Klapper J, Mathew N et al (2002) Intransal civamide for the treatment of episodic cluster headaches. Arch Neurol 59:990–994
Ansarinia M, Rezai A, Tepper S et al (2010) Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache 50:1164–1174
Rapoport A (2011) New frontiers in headache therapy. Neurol Sci 32:105–109
Conflict of interest
A. Rapoport is on the Advisory Boards of NuPathe and MAP, he consults for Autonoic Technologies, Inc and Winston and is an author of the Phase IIB study on telcagepant studied by Merck.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rapoport, A.M. The therapeutic future in headache. Neurol Sci 33 (Suppl 1), 119–125 (2012). https://doi.org/10.1007/s10072-012-1056-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-012-1056-3