Abstract
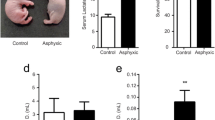
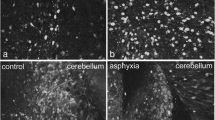
Molecular processes regulating cholinergic functions play an important role in the control of respiration under neonatal hypoxia. The present study evaluates neonatal hypoxic insult-mediated cholinergic alterations and the protective role of glucose, oxygen and epinephrine resuscitation. The changes in total muscarinic, muscarinic M1, M2, M3 receptors and the enzymes involved in acetylcholine metabolism––cholineacetyl transferase and acetylcholine easterase in the brain stem were analyzed. Hypoxic stress decreased total muscarinic receptors along with a reduction in muscarinic M1, M2 and M3 receptor genes in the brain stem. The reduction in acetylcholine metabolism is indicated by the down regulated cholineacetyl transferase and up regulated acetylcholine easterase expression. These cholinergic disturbances in the brain stem were reversed by glucose resuscitation to hypoxic neonates. The adverse effects of immediate oxygenation and epinephrine administration were also reported. This has immense clinical significance in establishing a proper resuscitation for the management of neonatal hypoxia.








Similar content being viewed by others
References
Alkondon M, Albuquerque EX (2006) Subtype-specific inhibition of nicotinic acetylcholine receptors by choline: A regulatory pathway. J Pharmacol Exp Therap 318:268–275
Ballantyne D, Scheid P (2000) Mammalian brainstem chemosensitive neurones: linking them to respiration in vitro. J Physiol 525(3):567–577
Beley A, Bertrand N, Beley P (1991) Cerebral ischemia: changes in brain choline, acetylcholine, and other monoamines as related to energy metabolism. Neurochem Res 16:555–561
Bellingham MC, Berger AJ (1996) Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol 76(6):3758–3770
Bellingham MC, Ireland MF (2002) Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol 131(1–2):135–144
Berg AT (1988) Childhood neurological morbidity and its association with gestational age, intrauterine growth retardation and perinatal stress. Paediatr Perinat Epidemiol 2:229–238
Boudinot E, Champagnat J, Foutz AS (2008) M1/M3 and M2/M4 muscarinic receptor double–knockout mice present distinct respiratory phenotypes. Respir Physiol Neurobiol 161:54–61
Burton MD, Kazemi H (2000) Neurotransmitters in central respiratory control. Respir Physiol 122(2–3):111–121
Burton MD, Nouri K, Baichoo S, Samuels–Toyloy N, Kazemi H (1994) Ventilatory output and acetylcholine: perturbations in release and muscarinic receptor activation. J Appl Physiol 77:2275–2284
Carroll JL (2003) Developmental plasticity in respiratory control. J Appl Physiol 94(1):375–389
Casolini P, Zuena AR, Cinque C, Matteucci P, Alemà GS, Adriani W, Carpinelli G, Santoro F, Alleva E, Bosco P, Nicoletti F, Laviola G, Catalani A (2005) Sub-neurotoxic neonatal anoxia induces subtle behavioural changes and specific abnormalities in brain group-I metabotropic glutamate receptors in rats. J Neurochem 95(1):137–145
Chang HM, Wei IH, Tseng CY, Lue JH, Wen CY, Shieh JY (2004) Differential expression of calcitonin gene-related peptide (CGRP) and choline acetyltransferase (ChAT) in the axotomized motoneurons of normoxic and hypoxic rats. Chem Neuroanat 28(4):239–251
Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW (2009) Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Respir Physiol Neurobiol 166(1):4–12
Cooper JR, Bloom FE, Roth RH (2003) Acetylcholine. The Biochemical Basis of Neuropharmacology Oxford University Press, New York
Darnall RA, Ariagno RL, Kinney HC (2006) The late preterm infant and the control of breathing, sleep, and brainstem development: a review. Clin Perinatol 33(4):883–914
du Plessis AJ, Volpe JJ (2002) Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol 15:151–157
Dutschmann M, Herbert H (1999) Pontine cholinergic mechanisms enhance trigeminally evoked respiratory suppression in the anesthetized rat. J Appl Physiol 87(3):1059–1065
Ellman GL, Courtney KD, Andres V Jr, Father-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Flavin MP, Yang Y, Ho G (1993) Hypoxic forebrain cholinergic neuron injury: role of glucose excitatory amino acid receptors and nitric oxide. Neurosci Lett 164:5–8
Freeman GB, Mykytyn V, Gibson GE (1987) Differential alteration of dopamine, acetylcholine and glutamate release during anoxia and/or 3,4- diaminopyridine treatment. Neurochem Res 12:1019–1027
Furchgott RF, Sleator W Jr, De GUbareff T (1960) Effects of acetylcholine and epinephrine on the contractile strength and action potential of electrically driven guinea pig atria. J Pharmacol Exp Ther 129:405–416
Gibbs RB (1999) Oestrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav 36:222–233
Gibson GE, Duffy TE (1981) Impaired Synthesis of Acetylcholine by Mild Hypoxic Hypoxia or Nitrous Oxide. J Neurochem 36(1):28–33
Gibson GE, Peterson C (1981) Decrease in acetylcholine release in vitro with low oxygen. Biochem Pharmacol 31:111–115
Gireesh G, Kaimal SB, Kumar TP, Paulose CS (2008) Decreased muscarinic M1 receptor gene expression in the hypothalamus, brainstem, and pancreatic islets of streptozotocin-induced diabetic rats. J Neurosci Res 86(4):947–953
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain: the disposition of [3H] Norepinephrine, [3H] DOPA in various regions of the brain. J Neurochem 13:655–669
Haji A, Takeda R, Okazaki M (2000) Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther 86:277–304
Kim MH, Kim MO, Heo JS, Kim JS, Han HJ (2008) Acetylcholine inhibits long-term hypoxia-induced apoptosis by suppressing the oxidative stress-mediated MAPKs activation as well as regulation of Bcl-2, c-IAPs, and caspase-3 in mouse embryonic stem cells. Apoptosis 13(2):295–304
Kouniniotou-Krontiri P, Tsakiris S (1989) Time dependence of Li + action on acetylcholinesterase activity in correlation with spontaneous quantal release of acetylcholine in rat diaphragm. Jpn J Physiol 39:429–440
Lai J, Shao XM, Pan RW, Dy E, Huang CH, Feldman JL (2001) RT-PCR reveals muscarinic acetylcholine receptor mRNA in the pre-Botzinger complex. Am J Physiol Lung Cell Mol Physiol 281:L1420–L1424
Lindahl E, Michelsson K, Helenius M, Parre M (1988) Neonatal risk factors and later neurodevelopmental disturbances. Dev Med Child Neurol 30:571–589
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Muthuraju S, Maiti P, Solanki P, Sharma AK, Pati S, Singh SB, Prasad D, Ilavazhagan G (2011) Possible role of cholinesterase inhibitors on memory consolidation following hypobaric hypoxia of rats. Int J Neurosci 121:279–288
Nattie EE, Li A (1990) Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J Appl Physiol 69:33–41
Nyakas C, Buwalda B, Luiten PG (1996) Hypoxia and brain development. Prog Neurobiol 49:1–51
Paulose CS, Chathu F, Khan SR, Krishnakumar A (2008) Neuroprotective role of Bacopa monnieri extract in epilepsy and effect of glucose supplementation during hypoxia: glutamate receptor gene expression. Neurochem Res 33(9):1663–1671
Peterson BS (2003) Brain imaging studies of the anatomical and functional consequences of preterm birth for human brain development. Ann NY Acad Sci. 1008:219–237
Renuka TR, Ani DV, Paulose CS (2004) Alterations in the muscarinic M1 and M3 receptor gene expression in the brain stem during pancreatic regeneration and insulin secretion in weanling rats. Life Sci 75(19):2269–2280
Rodrigo J, Fernandez AP, Serrano J, Peinado MA, Martinez A (2005) The role of free radicals in cerebral hypoxia and ischemia. Free Radic Biol Med 39:26–50
Rowland NE, Farnbauch LJ, Robertson KL (2003) Brain muscarinic receptor subtypes mediating water intake and Fos following cerebroventricular administration of bethanecol in rats. Psychopharmacology 167:174–179
Scatchard G (1949) The attractions of proteins for small molecules and ions. Ann N Y Acad Sci 51:660–672
Schubert S, Brandl U, Brodhun M (2005) Neuroprotective effects hypoxia—ischemia in newborn piglets. Brain Res 1058:129–136
Shao XM, Feldman JL (2000) Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in pre-Bötzinger complex inspiratory neurons. J Neurophysiol 83:1243–1252
Shah P, Riphagen S, Beyene J, Perlman JM (2004) Multiorgan dysfunction in infants with post-asphyxial hypoxic—ischemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 89:F152–F155
Soulier V, Peyronnet J, Pequignot JM, Cottet–Emard JM, Lagercrantz H, Dalmaz Y (1997) Long–term impairment in the neurochemical activity of the sympathoadrenal system after neonatal hypoxia in the rat. Pediatr Res 42:30–38
Tanaka H, Takahashi S, Miyamoto A, Oki J, Cho K, Okuno A (1995) Effects of neonatal hypoxia on brainstem cholinergic neurons-pedunculopontine nucleus and laterodorsal tegmental nucleus. Brain Dev 17:264–270
Unsworth BR, Fleming LH, Caron PC (1980) Neurotransmitter enzymes in telencephalon, brain stem and cerebellum during the entire life span of the mouse. Mech Ageing Dev 13:205–217
Vento M, Sastre J, Asensi MA, Vina J (2005) Room-air resusciatation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med 172:1393–1398
Weihua X, Judith AS, Arnaud C, Philip JW, Angie R, Rodney DM, Palmer T, Steven HH, Oksana L (2000) Postnatal developmental delay and supersensitivity to organophosphate in gene-targeted mice lacking acetylcholineesterase. Pharmacology 293:896–902
Willoughby J, Harvey SAK, John BC (1986) Compartmentation and regulation of acetyIcholine synthesis at the Synapse. Biochem J 235:215–223
Yamamura HI, Synder G (1981) Binding of [3H] QNB in rat brain. Proc Natl Acad Sci 71:1725–1729
Zapata A, Capdevila JL, Trullas R (1998) Region-specific and calciumdependent increase in dialysate choline levels by NMDA. J Neurosci 18:3597–3605
Acknowledgments
This work was supported by the research grants from DBT, DST, ICMR, Govt. of India and KSCSTE, Government of Kerala to Dr. C. S. Paulose. Anju T R thanks Council of Scientific and Industrial Research for Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anju, T.R., Naijil, G., Shilpa, J. et al. Neonatal hypoxic insult-mediated cholinergic disturbances in the brain stem: effect of glucose, oxygen and epinephrine resuscitation. Neurol Sci 34, 287–296 (2013). https://doi.org/10.1007/s10072-012-0989-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-012-0989-x