Abstract
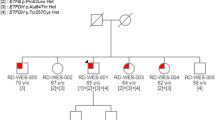
Multiple acyl-CoA dehydrogenase deficiency (MADD) is a rare autosomal recessively inherited disorder of fatty acid metabolism due to ETFA, ETFB or ETFDH mutations. Riboflavin treatment ameliorates symptoms and metabolic profile in ETFDH-related MADD patients. We report on a 20-year-old boy with an 8-year history of progressive difficulty in walking, running and climbing stairs. Muscle biopsy showed a lipid myopathy and the acylcarnitine profile analysis was suggestive of MADD. Nevertheless, no evidence of molecular defects in the ETFA, ETFB and ETFDH exons or intron–exon boundaries was found. Treatment with riboflavin for 1 year resulted in a clear improvement in motor functions. Our report shows that some cases of MADD are not linked to ETFA, ETFB and ETFDH exon or intron–exon boundary changes. They could be due to quite rare promoter or deep intronic mutations or, most likely, to some unknown genetic defect. We therefore suggest to extend in these cases molecular studies to cDNA analysis and indicate the need of extensive pedigree studies to identify other possible disease-related loci. Most important, treatment of these cases with riboflavin can also be effective. Therefore, early diagnosis is essential to achieve the best treatment response.

Similar content being viewed by others
References
Frerman FE, Goodman SI (2001) Defects of electron transfer flavoprotein and electron transfer flavoprotein ubiquinone oxidoreductase: glutaric acidemia type II. In: Scriver CR et al (eds) The metabolic and molecular bases of inherited diseases. McGraw-Hill, New York, pp 2357–2365
Liang WC, Nishino I (2001) Lipid storage myopathy. Curr Neurol Neurosci Rep 1:97–103
Yamaguchi S, Orii T, Maeda K, Oshima M, Hashimoto T (1990) A new variant of glutaric aciduria type II: deficiency of beta-subunit of electron transfer flavoprotein. J Inherit Metab Dis 13:783–786
Goodman S, Binard R, Woontner M, Frerman F (2002) Glutaric acidemia type II: gene structure and mutations of the electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO) gene. Mol Genet Metab 77:86–90
Olsen RK, Andresen BS, Christensen E, Bross P, Skovby F, Gregersen N (2003) Clear relationship between ETF/ETFDH genotype and phenotype in patients with multiple acyl-CoA dehydrogenation deficiency. Hum Mutat 22:12–23
Schiff M, Froissart R, Olsen RK, Acquaviva C, Vianey-Saban C (2006) Electron transfer flavoprotein deficiency: functional and molecular aspects. Mol Genet Metab 88:153–158
Gregersen N, Rhead W, Christensen E (1990) Riboflavin responsive glutaric aciduria type II. Prog Clin Biol Res 321:477–494
Rhead W, Roettger V, Marshall T, Amendt B (1993) Multiple acyl-coenzyme A dehydrogenation disorder responsive to riboflavin: substrate oxidation, flavin metabolism, and flavoenzyme activities in fibroblasts. Pediatr Res 33:129–135
Olsen RK, Olpin SE, Andresen BS et al (2007) ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain 130:2045–2054
Colevas AD, Edwards JL, Hruban RH, Mitchell GA, Valle D, Hutchins GM (1988) Glutaric acidemia type II. Comparison of pathologic features in two infants. Arch Pathol Lab Med 112:1133–1139
Angle B, Burton BK (2008) Risk of sudden death and acute life-threatening events in patients with glutaric acidemia type II. Mol Genet Metab 93:36–39
al-Essa MA, Rashed MS, Bakheet SM, Patay ZJ, Ozand PT (2000) Glutaric aciduria type II: observations in seven patients with neonatal- and late-onset disease. J Perinatol 20:120–128
Henriques BJ, Rodrigues JV, Olsen RK, Bross P, Gomes CM (2009) Role of flavinylation in a mild variant of multiple acyl-CoA dehydrogenation deficiency: a molecular rationale for the effects of riboflavin supplementation. J Biol Chem 284:4222–4229
Law LK, Tang NL, Hui J et al (2009) Novel mutations in ETFDH gene in Chinese patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin Chim Acta 404:95–99
Wen B, Dai T, Li W et al (2010) Riboflavin-responsive lipid-storage myopathy caused by ETFDH gene mutations. J Neurol Neurosurg Psychiatry 81:231–236
Gempel K, Topaloglu H, Talim B et al (2007) The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain 130:2037–2044
Liang WC, Ohkuma A, Hayashi YK et al (2009) ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord 19:212–216
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cotelli, M.S., Vielmi, V., Rimoldi, M. et al. Riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency with unknown genetic defect. Neurol Sci 33, 1383–1387 (2012). https://doi.org/10.1007/s10072-011-0900-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0900-1