Abstract
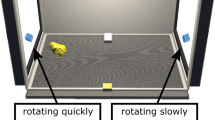
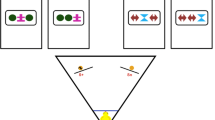
To what extent are newborn brains designed to operate over natural visual input? To address this question, we used a high-throughput controlled-rearing method to examine whether newborn chicks (Gallus gallus) show enhanced learning of natural visual sequences at the onset of vision. We took the same set of images and grouped them into either natural sequences (i.e., sequences showing different viewpoints of the same real-world object) or unnatural sequences (i.e., sequences showing different images of different real-world objects). When raised in virtual worlds containing natural sequences, newborn chicks developed the ability to recognize familiar images of objects. Conversely, when raised in virtual worlds containing unnatural sequences, newborn chicks’ object recognition abilities were severely impaired. In fact, the majority of the chicks raised with the unnatural sequences failed to recognize familiar images of objects despite acquiring over 100 h of visual experience with those images. Thus, newborn chicks show enhanced learning of natural visual sequences at the onset of vision. These results indicate that newborn brains are designed to operate over natural visual input.





Similar content being viewed by others
Notes
Due to an equipment malfunction, we were only able to analyze the imprinting rates from the input phase for half of the subjects (the subjects raised with the sequences shown in Panel B in Fig. 1).
References
Chiandetti C, Vallortigara G (2011) Intuitive physical reasoning about occluded objects by inexperienced chicks. Proc Biol Sci 278:2621–2627
Cook RG, Roberts S (2007) The role of video coherence on object-based motion discriminations by pigeons. J Exp Psychol Anim Behav Proc 33:287–298
Cook RG, Shaw R, Blaisdell AP (2001) Dynamic object perception by pigeons: discrimination of action in video presentations. Anim Cogn 4:137–146
Cox DD, Meier P, Oertelt N, DiCarlo JJ (2005) ‘Breaking’ position-invariant object recognition. Nat Neurosci 8:1145–1147
DiCarlo JJ, Zoccolan D, Rust NC (2012) How does the brain solve visual object recognition? Neuron 73:415–434
Duncan K, Sadanand A, Davachi L (2012) Memory’s penumbra: episodic memory decisions induce lingering mnemonic biases. Science 337:485–487
Foldiak P (1991) Learning invariance from transformation sequences. Neural Comput 3:194–200
Goldman JG, Wood JN (2015) An automated controlled-rearing method for studying the origins of movement recognition in newly hatched chicks. Anim Cogn 18:723–731
Hasselmo ME, Schnell E (1994) Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region Ca1: computational modeling and brain slice physiology. J Neurosci 14:3898–3914
Horn G (2004) Pathways of the past: the imprint of memory. Nat Rev Neurosci 5:108–120
Hung CP, Kreiman G, Poggio T, DiCarlo JJ (2005) Fast readout of object identity from macaque inferior temporal cortex. Science 310:863–866
Jarvis ED et al (2005) Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci 6:151–159
Karten HJ (2013) Neocortical evolution: neuronal circuits arise independently of lamination. Curr Biol 23:R12–R15
Lades M, Vorbruggen JC, Buhmann J, Lange J, Vandermalsburg C, Wurtz RP, Konen W (1993) Distortion invariant object recognition in the dynamic link architecture. IEEE Trans Comput 42:300–311
Li N, DiCarlo JJ (2008) Unsupervised natural experience rapidly alters invariant object representation in visual cortex. Science 321:1502–1507
Li N, DiCarlo JJ (2010) Unsupervised natural visual experience rapidly reshapes size-invariant object representation in inferior temporal cortex. Neuron 67:1062–1075
Mascalzoni E, Osorio D, Regolin L, Vallortigara G (2012) Symmetry perception by poultry chicks and its implications for three-dimensional object recognition. Proc Biol Sci 279:841–846
Masquelier T, Thorpe SJ (2007) Unsupervised learning of visual features through spike timing dependent plasticity. PLoS Comput Biol 3:247–257
O’Reilly RC, McClelland JL (1994) Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus 4:661–682
Regolin L, Vallortigara G (1995) Perception of partly occluded objects by young chicks. Percept Psychophys 57:971–976
Rugani R, Fontanari L, Simoni E, Regolin L, Vallortigara G (2009) Arithmetic in newborn chicks. Proc Biol Sci 276:2451–2460
Serre T, Oliva A, Poggio T (2007) A feedforward architecture accounts for rapid categorization. Proc Natl Acad Sci USA 104:6424–6429
Shanahan M, Bingman VP, Shimizu T, Wild M, Gunturkun O (2013) Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front Comput Neurosci 7:89
Stone JV (1996) Learning perceptually salient visual parameters using spatiotemporal smoothness constraints. Neural Comput 8:1463–1492
Wallis G (2013) Toward a unified model of face and object recognition in the human visual system. Front Psychol 4:1–25
Wallis G, Bulthoff HH (2001) Effects of temporal association on recognition memory. Proc Natl Acad Sci USA 98:4800–4804
Wallis G, Rolls ET (1997) Invariant face and object recognition in the visual system. Prog Neurobiol 51:167–194
Wiskott L, Sejnowski TJ (2002) Slow feature analysis: unsupervised learning of invariances. Neural Comput 14:715–770
Wood JN (2013) Newborn chickens generate invariant object representations at the onset of visual object experience. Proc Natl Acad Sci USA 110:14000–14005
Wood JN (2014) Newly hatched chicks solve the visual binding problem. Psychol Sci 25:1475–1481
Wood JN (2015) Characterizing the information content of a newly hatched chick’s first visual object representation. Dev Sci 18:194–205
Wood SM, Wood JN (2015a) A chicken model for studying the emergence of invariant object recognition. Front Neural Circuits 9:7
Wood SMW, Wood JN (2015b) Face recognition in newly hatched chicks at the onset of vision. J Exp Psychol Anim Learn Cogn 41:206–215
Wyss R, Konig P, Verschure PFMJ (2006) A model of the ventral visual system based on temporal stability and local memory. PLoS Biol 4:836–843
Xu X, Biederman I (2010) Loci of the release from fMRI adaptation for changes in facial expression, identity, and viewpoint. J Vis 10:1–13
Yamins DLK, Hong H, Cadieu CF, Solomon EA, Seibert D, DiCarlo JJ (2014) Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc Natl Acad Sci USA 111:8619–8624
Acknowledgments
This research was funded by National Science Foundation CAREER Grant BCS-1351892. Stimulus images courtesy of Michael J. Tarr, Center for the Neural Basis of Cognition and Department of Psychology, Carnegie Mellon University, http://www.tarrlab.org/. We thank Brian W. Wood for assistance with the supplementary movies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 12191 kb)
Supplementary material 2 (MOV 12303 kb)
Rights and permissions
About this article
Cite this article
Wood, J.N., Prasad, A., Goldman, J.G. et al. Enhanced learning of natural visual sequences in newborn chicks. Anim Cogn 19, 835–845 (2016). https://doi.org/10.1007/s10071-016-0982-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-016-0982-5