Abstract
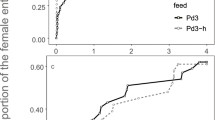
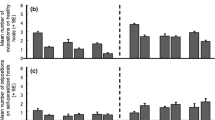
Parasitic wasps are faced with the decision of where and for how long to search for hosts. Their leaving decisions depend on the rate at which new host-containing patches are encountered: parasitoids increase foraging efficiency by leaving earlier when patch encounter rates become higher. The mechanisms by which these often tiny insects can assess patch encounter rates have not been thoroughly investigated so far. The aim of the present study, where females of the braconid wasp Asobara tabida encountered patches after varying time intervals, was to measure the shape of the travel–time response curve and to analyse how information on inter-patch distances is translated into foraging behaviour. I examined several proxies for travel-time duration, like those of physiological nature as egg content, cues of senescence, amount of energy spent, or muscle fatigue, as well as true cognitive mechanisms, like measurement of distance or interval timing. Constraints in the wasp’s ability to detect patch borders accurately after travelling, e.g. habituation to the patch odour or receptor blocking, are also discussed. From the data presented, most of the above-mentioned mechanisms and constraints can be rejected to work for A. tabida. The effects of inter-patch travel time are strongest when they are short, and even though it cannot be excluded that time measures are processed using an internal clock, I suggest that a Bayesian-like mechanism of timing, the biological basis of which might involve the build-up of neurosecretory material, is the most likely candidate influencing leaving decisions in A. tabida.








Similar content being viewed by others
References
Adler FR, Kotar M (1999) Departure time versus departure rate: how to forage optimally when you are stupid. Evol Ecol Res 1:411–421
Aparicio CF, Baum WM (1997) Comparing locomotion with lever-press travel in an operant simulation of foraging. J ExpAnal Behav 68:177–192
Bateson M (2003) Interval timing and optimal foraging: functional and neural mechanisms of interval timing. In: Meck WH (ed) CRC Press, Boca Raton
Bateson M, Kacelnik A (1995) Accuracy of memory for amount in the foraging starling (Sturnus vulgaris). Anim Behav 50:431–443
Bizo LA, Chu JYM, Sanabria F, Killeen PR (2006) The failure of Weber’s law in time perception and production. Behav Proc 71:201–210
Boisvert MJ, Sherry DF (2006) Interval timing by an invertebrate, the bumble bee Bombus impatiens. Cur Biol 16:1636–1640
Boivin G, Fauvergue X, Wajnberg E (2004) Optimal patch residence time in egg parasitoids: innate versus learned estimate of patch quality. Oecologia 138:640–647
Brunner D, Kacelnik A, Gibbon J (1992) Optimal foraging and timing processes in the starling, Sturnus vulgaris—effect of inter-capture interval. Anim Behav 44:597–613
Brunner D, Kacelnik A, Gibbon J (1996) Memory for inter-reinforcement interval variability and patch departure decisions in the starling, Sturnus vulgaris. Anim Behav 51:1025–1045
Cassini MH, Lichtenstein G, Ongay JP, Kacelnik A (1993) Foraging behavior in guinea-pigs—further tests of the marginal value theorem. Behav Proc 29:99–112
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theoret Pop Biol 9:129–136
Cheng K, Srinivasan MV, Zhang SW (1999) Error is proportional to distance measured by honeybees: Weber’s law in the odometer. Anim Cogn 2:11–16
Collins MD, Dixon AFG (1986) The effect of egg depletion on the foraging behaviour of an aphid parasitoid. J Appl Ent 102:342–352
Cowie RJ (1977) Optimal foraging in great tits (Parus major). Nature 268:137–139
Crawley MJ (2005) Statistics. An introduction using R. Wiley, New York
Cuthill IC, Haccou P, Kacelnik A (1994) Starlings (Sturnus vulgaris) exploiting patches: response to long term changes in travel time. Behav Ecol 5:81–90
Dacke M, Srinivasan MV (2007) Honeybee navigation: distance estimation in the third dimension. J Exp Biol 210:845–853
Desouhant E, Driessen G, Amat I, Bernstein C (2005) Host and food searching in a parasitic wasp Venturia canescens: a trade-off between current and future reproduction? Anim Behav 70:145–152
Ellers J (1997) Life history evolution in Asobara tabida: plasticity in allocation of fat reserves to survival and reproduction. J Evol Biol 10:771–785
Ellers J, van Alphen JJM, Sevenster JG (1998) A field study of size-fitness relationships in the parasitoid Asobara tabida. J Anim Ecol 67:318–324
Ellers J, Driessen G, Sevenster JG (2000) The shape of the trade-off function between egg production and life span in the parasitoid Asobara tabida. Neth J Zooly 50:29–36
Galis F, van Alphen JJM (1981) Patch time allocation and search intensity of Asobara tabida Nees (Braconidae), a larval parasitoid of Drosophila. Neth J Zool 31:596–611
Gibbon J (1991) Origins of scalar timing. Learn Motiv 22:3–38
Godfray HCJ (1994) Parasitoids. Behavioral and evolutionary ecology. Princeton University Press, Princeton
Godfray HCJ, Waage JK (1988) Learning in parasitic wasps. Nature 331:211
Grafen A, Hails R (2002) Modern statistics for the life sciences
Haccou P, de Vlas SJ, van Alphen JJM, Visser ME (1991) Information processing by foragers: effects of intra-patch experience on the leaving tendency of Leptopilina heterotoma. J Anim Ecol 60:93–106
Hemerik L, Driessen G, Haccou P (1993) Effects of intra-patch experiences on patch time, search time and searching efficiency of the parasitoid Leptopilina clavipes. J Anim Ecol 62:33–44
Hoffmeister TS, Rohlfs M (2001) Aggregative egg distributions may promote species co-existence—but why do they exist? Evol Ecol Res 3:37–50
Howse P (1975) Brain structure and behavior in insects. Ann Rev Entomol 20:359–379
Iwasa Y, Higashi M, Yamamura N (1981) Prey distribution as a factor determining the choice of optimal foraging strategy. Am Nat 117:710–723
Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NAC (2001) Life-history strategies in parasitoid wasps: a comparative analysis of ‘ovigeny’. J Anim Ecol 70:442–458
Jordan KE, Brannon EM (2006) Weber’s Law influences numerical representations in rhesus macaques (Macaca mulatta). Anim Cogn 9:159–172
Kacelnik A, Todd IA (1992) Psychological mechanisms and the marginal value theorem: effect of variability in travel time on patch exploitation. Anim Behav 43:313–322
Kaissling KE (2001) Olfactory perireceptor and receptor events in moths: a kinetic model. Chem Senses 26:125–150
Kawamori A, Matsushima T (2010) Subjective value of risky foods for individual domestic chicks: a hierarchical Bayesian model. Anim Cogn 13:431–441
Lange A, Dukas R (2009) Bayesian approximations and extensions: optimal decisions for small brains and possibly big ones too. J Theoret Biol 259:503–516
Lefebvre D, Pierre J, Outreman Y, Pierre JS (2007) Patch departure rules in Bumblebees: evidence of a decremental motivational mechanism. Behav Ecol Sociobiol 61:1707–1715
Lewis PA, Miall RC (2009) The precision of temporal judgement: milliseconds, many minutes, and beyond. Phil Trans R Soc B 364:1897–1905
Liu Y-Q, Bernstein C, Thiel A (2009) Travel duration, energetic expenditure, and patch exploitation in the parasitic wasp Venturia canescens. Behav Ecol Sociobiol 63:1459–1469. doi:10.1007/s00265-009-0800-z
Lucchetta P, Desouhant E, Wajnberg E, Bernstein C (2007) Small but smart: the interaction between environmental cues and internal state modulates host-patch exploitation in a parasitic wasp. Behav Ecol Sociobiol 61:1409–1418
Mangel M (1993) Motivation, learning, and motivated learning: insect learning: ecological and evolutionary perspectives. In: Papaj DR, Lewis AC (eds) Chapman & Hall, New York, pp 158–173
Matell MS, Meck WH (2000) Neuropsychological mechanisms of interval timing behavior. Bioessays 22:94–103
Mc Namara JM, Houston AI (1985) Optimal foraging and learning. J Theoret Biol 117:231–249
Mc Namara JM, Houston AI (1987) Memory and the efficient use of information. J Theoret Biol 125:385–395
Mc Namara JM, Green RF, Olsson O (2006) Bayes’ theorem and its applications in animal behaviour. Oikos 112:243–251
Menzel R (1999) Memory dynamics in the honeybee. J Comp Physiol A 185:323–340
Mizunami M, Unoki S, Mori Y, Hirashima D, Hatano A, Matsumoto Y (2009) Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol 7:46
Muratori F, Boivin G, Hance T (2008) The impact of patch encounter rate on patch residence time of female parasitoids increases with patch quality. Ecol Entomol 33:422–427
Nelder JA, Wedderburn RW (1972) Generalized linear models. J Royal Stat Soc A 135:370–384
Ollason JG (1980) Learning to forage—optimally? Theoret Pop Biol 18:44–56
Peeke HVS, Herz MJ (1973) Habituation. Volume I: Behavioral studies. Academic Press, New York
Pierre JS, Green RF (2008) A Bayesian approach to optimal foraging in parasitoids. In: Wajnberg E, Bernstein C, van Alphen JJM (eds) Behavioral ecology of insect parasitoids. Blackwell, London, pp 357–383
Pierre JS, van Baaren J, Boivin G (2003) Patch leaving decision rules in parasitoids: do they use sequential decisional sampling? Behav Ecol Sociobiol 54:147–155
Reboreda JC, Kacelnik A (1991) Risk sensitivity in starlings: variability in food amount and food delay. Behav Ecol 2:301–308
Roitberg BD, Prokopy RJ (1982) Influence of intertree distance on foraging behaviour of Rhagoletis pomonella in the field. Ecol Entomol 7:437–442
Rosenberg L, Glusman J, Libersat F (2007) Octopamine partially restores walking in hypokineticcockroaches stung by the parasitoid wasp Ampulex compressa. J Exp Biol 210:4411–4417
SAS Institute Inc (1999) SAS OnlineDoc®. SAS Institute Inc, Cary
Schmidt JM, Smiths JJB (1985) The mechanism by which the parasitoid Trichogramma minutum responds to host clusters. Entomol Exp Appl 39:287–294
Schmidt JM, Smiths JJB (1987) Short interval time measurement by a parasitoid wasp. Science 237:903–905
Shettleworth SJ (1998) Cognition, evolution and behavior. Oxford University Press, Inc, Oxford
Sirot E, Bernstein C (1997) Food searching and superparasitism in solitary parasitoids. Acta Oecol Int J Ecol 18:63–72
Stephens DW (1993) Learning and behavioral ecology: incomplete information and environmental predictability. In: Papaj DR, Lewis AC (eds) Insect learning: ecological and evolutionary perspectives. Chapman & Hall, New York, pp 195–218
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Tentelier C, Desouhant E, Fauvergue X (2006) Habitat assessment by parasitoids: mechanisms for patch use behavior. Behav Ecol 17:515–521
Thiel A (2000) Zum Wirtssuchverhalten von Drosophila-Parasitoiden. Diploma thesis, Christian-Albrechts-University Kiel
Thiel A (2004) Die Bedeutung von Habitatparametern für das Suchverhalten parasitischer Wespen. Dissertation, Christian-Albrechts-University Kiel
Thiel A, Hoffmeister TS (2004) Knowing your habitat: linking patch encounter rate and patch exploitation in parasitoids. Behav Ecol 15:419–425
Thiel A, Hoffmeister TS (2006) Selective information use in parasitoid wasps. Anim Biol 56:233–245
Thiel A, Driessen G, Hoffmeister TS (2006) Different habitats, different habits? Response to foraging information in the parasitic wasp Venturia canescens. Behav Ecol Sociobiol 59:614–623. doi:10.1007/s00265-005-0088-6
Tobin S, Bisson N, Grondin S (2010) An ecological approach to prospective and retrospective timing of long durations: a study involving gamers. PLoS ONE 5:e9271. doi:10.1371/journal.pone.0009271
Todd IA, Kacelnik A (1993) Psychological mechanisms and the marginal value theorem: dynamics of scalar memory for travel time. Anim Behav 46:765–775
Valone TJ (2006) Are animals capable of Bayesian updating? An empirical review. Oikos 112:252–259
van Alphen JJM, Bernstein C (2008) Information acquisition, information processing, and patch time allocation in insect parasitoids. In: Wajnberg E, Bernstein C, van Alphen JJM (eds) Behavioral ecology of insect parasitoids. Blackwell, London, pp 172–192
van Alphen JJM, Drijver RAB (1982) Host selection by Asobara tabida Nees (Braconidae; Alysiinae), a larval parasitoid of fruit inhabiting Drosophila species. I. Host stage selection with Drosophila melanogaster as host species. Neth J Zool 32:215–231
van Alphen JJM, Galis F (1983) Patch time allocation and parasitization efficiency of Asobara tabida, a larval parasitoid of Drosophila. J Anim Ecol 52:937–952
van Alphen JJM, Bernstein C, Driessen G (2003) Information acquisition and time allocation in insect parasitoids. Trends Ecol Evol 18:81–87
van Lenteren JC, Bakker K (1976) Functional responses in invertebrates. Neth J Zool 26:567–572
Visser ME, van Alphen JJM, Nell HW (1992) Adaptive superparasitism and patch time allocation in solitary parasitoids: the influence of pre-patch experience. Behav Ecol Sociobiol 31:163–171
Vogel H, Heinzeller T, Renner M (1977) Daily protein synthesis in the pars intercerebralis and corpora allata of Apis mellifica L. with and without training to a foraging schedule. J Comp Physiol 118:51–60
Waage JK (1979) Foraging for patchily-distributed hosts by the parasitoid, Nemeritis canescens. J Anim Ecol 48:353–371
Wajnberg E (2006) Time allocation strategies in insect parasitoids: from ultimate predictions to proximate behavioral mechanisms. Behav Ecol Sociobiol 60:589–611
Wajnberg E, Rosi MC, Colazza S (1999) Genetic variation in patch time allocation in a parasitic wasp. J Anim Ecol 68:121–133
Wyers EJ, Peeke HVS, Herz MJ (1973) Behavioral habituation in invertebrates: habituation. In: Peeke HVS, Herz MJ (eds) Volume I: Behavioral studies. Academic Press, New York, pp 1–57
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice-Hall, Englewood Cliffs
Acknowledgments
I am grateful to Joan van Baaren, Carlos Bernstein, Hans Smid, Thomas Hoffmeister, Hendrik Thiel and two anonymous referees for discussion and helpful comments on an earlier version of this manuscript. Ina Berndt is thanked for her help with the parasitoid culture and Thomas Hoffmeister for statistical advice. This work has been supported by DFG grant HO 1251/7-1. The experiments performed comply with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiel, A. How to measure patch encounter rate: decision-making mechanisms in the parasitic wasp Asobara tabida . Anim Cogn 14, 73–82 (2011). https://doi.org/10.1007/s10071-010-0344-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-010-0344-7