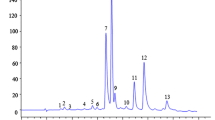
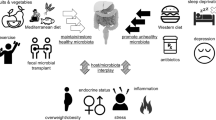
Abstract
Tea catechins have attracted strong interests in pharmacological field for their extensive biological activities; however, their bioavailability in vivo is relatively low. Recent studies have shown tea catechins can modulate the composition of intestinal microbiota and help to improve hosts’ health. Meanwhile, the gut flora plays a crucial role in regulating the production of the metabolites of tea catechins and their biological activity. Although the activities of tea catechins to promote intestinal micro-ecology have been extensively studied, little is known about the two-way phenol-microbial interactions. This review focuses on the modulatory effect of tea catechins on intestinal microbiota as well as the microbial degradation of tea catechins and the metabolites formed. Finally, the potential effects of tea catechins on chronic intestinal inflammation are emphasized.




Similar content being viewed by others
References
Bancirova M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. 43: 1379-1382 (2010)
Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sanchez E, Nabavi SF, Nabavi SM. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 196: 44-68 (2017)
Barnett MP, Cooney JM, Dommels YE, Nones K, Brewster DT, Park Z, Butts CA, Mcnabb WC, Laing WA, Roy NC. Modulation of colonic inflammation in Mdr1a(−/−) mice by green tea polyphenols and their effects on the colon transcriptome and proteome. J. Nutr. Biochem. 24: 1678-1690 (2013)
Bhooshan PK, Ibrahim RS. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2: 270-278 (2009)
Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 18: 715-723 (2017)
Chen T, Liu AB, Sun S, Ajami NJ, Ross MC, Wang H, Zhang L, Reuhl K, Kobayashi K, Onishi JC, Zhao L, Yang CS. Green tea polyphenols modify the gut microbiome in db/db mice as co-abundance groups correlating with the blood glucose lowering effect. Mol. Nutr. Food Res. 63: e1801064 (2019)
Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 59: 11862-11871 (2011)
Cheng M, Zhang X, Guo XJ, Wu ZF, Weng PF. The interaction effect and mechanism between tea polyphenols and intestinal microbiota: Role in human health. J. Food Biochem. 41: e12415 (2017)
Cheng M, Zhang X, Zhu J, Cheng L, Cao J, Wu Z, Weng P, Zheng X. A metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity mice fed with oolong tea polyphenols. Food Funct. 9: 1079-1087 (2018)
Chiou YS, Wu JC, Huang Q, Shahidi F, Wang YJ, Ho CT, Pan MH. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 7: 3-25 (2014)
Čitar M, Hacin B, Tompa G, Štempelj M, Rogelj I, Dolinšek J, Narat M, Matijašić BB. Human intestinal mucosa-associated Lactobacillus and Bifidobacterium strains with probiotic properties modulate IL-10, IL-6 and IL-12 gene expression in THP-1 cells. Benef. Microbes 6: 325-336 (2015)
Cristancho AG and Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12: 722-734 (2011)
Daglia M, Di Lorenzo A, Nabavi SF, Talas ZS, Nabavi SM. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Curr. Pharm. Biotechnol. 15: 362-372 (2014)
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559-563 (2014)
de Vadder F, Mithieux G. Gut-brain signaling in energy homeostasis: the unexpected role of microbiota-derived succinate. J. Endocrinol. 236: R105-R108 (2018)
Delzenne NM, Cani PD. Gut microbiota and the pathogenesis of insulin resistance. Curr. Diab. Rep. 11: 154-159 (2011)
Devadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156: 84-96 (2014)
Geremia A, Biancheri P, Allan P, Corazza GR, Di SA. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 13: 3-10 (2014)
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell 159: 789-799 (2014)
Guan F, Liu AB, Li G, Yang Z, Sun Y, Yang CS, Ju J. Deleterious effects of high concentrations of (-)-epigallocatechin-3-gallate and atorvastatin in mice with colon inflammation. Nutr. Cancer 64: 847-855 (2012)
Hänninen A1, Toivonen R, Pöysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 67: 1445-1453 (2018)
Hervert-Hernández D, Goñi I. Dietary polyphenols and human gut microbiota: a review. Food Rev. Int. 27: 154-169 (2011)
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486: 207-214 (2012)
Jin JS, Touyama M, Hisada T, Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 56: 729-739 (2012)
Juan MA, Lurdes B, Pilar A, Eider L, Javier M, Idoia L. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 111: 1263-1271 (2014)
Karri S, Sharma S, Hatware K, Patil K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 110: 224-238 (2019)
Kaulmann A, Bohn T. Bioactivity of polyphenols: preventive and adjuvant strategies toward reducing inflammatory bowel diseases-promises, perspectives, and pitfalls. Oxid. Med. Cell. Longev. 2016: 9346470 (2016)
Koh A, De VF, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165: 1332-1345 (2016)
Kohri T, Matsumoto N, Yamakawa M, Suzuki M, Nanjo F, Hara Y, Oku N. Metabolic fate of (−)-[4-3H]epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 49: 4102-4112 (2001)
Kutschera M, Engst W, Blaut M, Braune A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 111: 165-175 (2011)
Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 157: 876-884 (2006)
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022-1023 (2006)
Liao ZL, Zeng BH, Wang W, Li GH, Wu F, Wang L, Zhong QP, Wei H, Fang X. Impact of the consumption of tea polyphenols on early atherosclerotic lesion formation and intestinal Bifidobacteria in high-fat-fed ApoE−/− mice. Front. Nutr. 3: 42 (2016)
Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 53: 465-474 (2018)
Okubo H, Nakatsu Y, Kushiyama A, Yamamotoya T, Matsunaga Y, Inoue MK, Fujishiro M, Sakoda H, Ohno H, Yoneda M, Ono H, Asano T. Gut microbiota as a therapeutic target for metabolic disorders. Curr. Med. Chem. 25: 984-1001 (2018)
Oz HS, Chen T, de Villiers WJ. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front. Immunol. 4: 132 (2013)
Pan MH, Tung YC, Yang G, Li S, Ho CT. Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct. 7: 4481-4491 (2016)
Park JY, Chung TW, Jeong YJ, Kwak CH, Ha SH, Kwon KM, Abekura F, Cho SH, Lee YC, Ha KT, Magae J, Chang YC, Kim CH. Ascofuranone inhibits lipopolysaccharide-induced inflammatory response via NF-kappaB and AP-1, p-ERK, TNF-α, IL-6 and IL-1β in RAW 264.7 macrophages. Plos One 12: e0171322 (2017)
Pastoriza S, Mesías M, Cabrera C, Rufián-Henares JA. Healthy properties of green and white teas: an update. Food Funct. 8: 2650-2662 (2017)
Peery AF, Keku TO, Addamo C, McCoy AN, Martin CF, Galanko JA, Sandler RS. Colonic diverticula are not associated with mucosal inflammation or chronic gastrointestinal symptoms. Clin. Gastroenterol. Hepatol. 16: 884-891 (2018)
Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 64: 2847-2858 (2015)
Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nature 555: 210-215 (2018)
Rutgeerts P, Vermeire S, Assche GV. Biological therapies for inflammatory bowel diseases. Gastroenterology 136: 1182-1197 (2009)
Sheng L, Jena PK, Liu HX, Hu Y, Nagar N, Bronner DN, Settles ML, Bäumler AJ, Wan YY. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 8: fj201800370R (2018)
Stapleton PD, Shah S, Ehlert K, Hara Y, Taylor PW. The beta-lactam-resistance modifier (-)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 153: 2093-2103 (2007)
Takagaki A, Nanjo F. Catabolism of (+)-catechin and (−)-epicatechin by rat intestinal microbiota. J. Agric. Food Chem. 61: 4927-4935 (2013)
Takagaki A, Nanjo F. Metabolism of (−)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 58: 1313-1321 (2010)
Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 361: k2179 (2018)
Varilek GW, Yang F, Lee EY, de Villiers WJ, Zhong J, Oz HS, Westberry KF, McClain CJ. Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J. Nutr. 131: 2034-2039 (2001)
Williamson G, Clifford MN. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 139: 24-39 (2017)
Williamson G, Kay CD, Crozier A. The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Compr. Rev. Food Sci. F. 17: 1054-1112 (2018)
Wlodarska M, Kostic AD, Xavier RJ. An integrative view of microbiome-host interactions in inflammatory bowel diseases. Cell Host Microbe 17: 577-591 (2015)
Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu. Rev. Nutr. 33: 161-181 (2013)
Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 60: 528-533 (2001)
Yang Y, Qiao L, Zhang X, Wu Z, Weng P. Effect of methylated tea catechins from Chinese oolong tea on the proliferation and differentiation of 3T3-L1 preadipocyte. Fitoterapia 104: 45-49 (2015)
Zhang M, Merlin D. Nanoparticle-based oral drug delivery systems targeting the colon for treatment of ulcerative colitis. Inflamm. Bowel Dis. 24: 1401-1415 (2018)
Zhang M, Zhang X, Ho CT, Huang Q. Chemistry and health effect of tea polyphenol (−)-epigallocatechin 3-O-(3-O-methyl)gallate. J. Agric. Food Chem. 67: 5374-5378 (2019)
Zhang X, Chen Y, Zhu J, Zhang M, Ho CT, Huang Q, Cao J. Metagenomics analysis of gut microbiota in a high fat diet-induced obesity mouse model fed with (−)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3’’Me). Mol. Nutr. Food Res. 62: e1800274 (2018)
Zhang X, Zhu X, Sun Y, Hu B, Sun Y, Jabbar S, Zeng X. Fermentation in vitro of EGCG, GCG and EGCG3”Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 54: 1589-1595 (2013)
Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 33: 197-201 (2017)
Acknowledgements
This work was sponsored by Zhejiang Provincial Natural Science Foundation of China (LY19C200006), the Key Research and Development Project of Zhejiang Province (2017C02039 and 2018C02047), and K.C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, T., Song, D., Cheng, L. et al. Interactions of tea catechins with intestinal microbiota and their implication for human health. Food Sci Biotechnol 28, 1617–1625 (2019). https://doi.org/10.1007/s10068-019-00656-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00656-y