Abstract
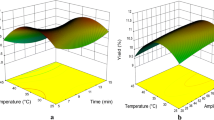
A Box–Behnken design (Extraction-time, pulse-cycle, sonication-amplitude) was employed to extract phenolic compounds from Justicia spicigera leaves by ultrasonic-assisted extraction. The muicle leaves extracts were analyzed measuring total phenolic compounds and antioxidant capacity. According to response surface methodology the optimal conditions of ultrasonic-assisted extraction to obtain the highest soluble phenolic content were 2 min (extraction time) for 0.7 s (pulse cycle) at 55% of sonication amplitude. Under these optimal conditions, the total phenolic content was higher when was used ultrasonic-assisted extraction (54.02 mg/g) than stirring (46.46 mg/g) and thermal decoction (47.76 mg/g); however, the antioxidant capacity from J. spicigera extracts did not increase by ultrasonic-assisted extraction. The extracts or aqueous infusions from J. spicigera leaves are used for therapeutic proposes, therefore the ultrasonic-assisted extraction is a useful technology to improve the extraction of phytochemicals from J. spicigera leaves.

Similar content being viewed by others
References
Sengupta SD, Paul R. Exploration of knowledge on new ethno-botanical value of Justicia spicigera Schltdl. Global J Res. Med. Plants Indigen. Med. 5: 261–266 (2016).
Pavón‐García LMA, Pérez‐Alonso C, Orozco‐Villafuerte J, Pimentel‐González DJ, Rodríguez‐Huezo ME, Vernon‐Carter EJ. Storage stability of the natural colourant from Justicia spicigera microencapsulated in protective colloids blends by spray‐drying. Int. J. Food Sci. Technol. 46: 1428–1437 (2011).
Zapata‐Morales JR, Alonso‐Castro AJ, Domínguez F, Carranza‐Álvarez C, Castellanos LMO, Martínez‐Medina RM, Pérez‐Urizar J. Antinociceptive Activity of an Ethanol Extract of Justicia spicigera. Drug Dev. Res. 77: 180–186 (2016).
Ortiz-Andrade R, Cabañas-Wuan A, Arana-Argáez VE, Alonso-Castro AJ, Zapata-Bustos R, Salazar-Olivo LA, Domínguez F, Chávez M, Carranza-Álvarez C, García-Carrancá A. Antidiabetic effects of Justicia spicigera Schltdl (Acanthaceae). J. Ethnopharma. 143: 455–462 (2012).
Esquivel-Gutiérrez ER, Noriega-Cisneros R, Arellano-Plaza M, Ibarra-Barajas M, Salgado-Garciglia R, Saavedra-Molina A. Antihypertensive effect of Justicia spicigera in L-NAME-induced hypertensive rats. Pharmacology. 2: 120–127 (2013).
Alonso-Castro AJ, Ortiz-Sánchez E, Domínguez F, Arana-Argáez V, Juárez-Vázquez MC, Chávez M, Carranza-Álvarez C, Gaspar-Ramírez O, Espinoza-Reyes G, López-Toledo G, Ortíz-Andrade R, García-Carrancá A. Antitumor and immunomodulatory effects of Justicia spicigera Schltdl (Acanthaceae). J. Ethnopharmacol. 141: 888–894 (2012).
Cassani J, Dorantes-Barrón AM, Novales LM, Real GA, Estrada-Reyes R. Anti-depressant-like effect of Kaempferitrin isolated from Justicia spicigera Schltdl (Acanthaceae) in two behavior models in mice: Evidence for the involvement of the serotonergic system. Molecules. 19: 21442–21461 (2014).
Sepúlveda-Jiménez G, Reyna-Aquino C, Chaires-Martínez L, Bermúdez-Torres K, Rodríguez-Monroy M. Antioxidant activity and content of phenolic compounds and flavonoids from Justicia spicigera. J. Biol. Sci. 9: 629–632 (2009).
Baqueiro-Peña I, Guerrero-Beltrán JA. Uses of Justicia spicigera in medicine and as a source of pigments. Funct. Foods Heal. Dis. 4: 401–414 (2014).
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 117: 426–436 (2013).
Zamora‐Gasga VM, Serafín‐García MS, Sánchez‐Burgos JA, Estrada RMV, Sáyago‐Ayerdi SG. Optimization of Ultrasonic‐Assisted Extraction of antioxidant compounds from Starfruit (Averroha carambola L) leaves. J. Food Process. Preserv. https://doi.org/10.1111/jfpp.13093 (2016).
Upadhyay R, Nachiappan G, Mishra HN. Ultrasound-assisted extraction of flavonoids and phenolic compounds from Ocimum tenuiflorum leaves. Food Sci. Biotechnol. 24: 1951–1958 (2015).
Baqueiro-Peña I, Guerrero-Beltrán JA. Physicochemical and antioxidant characterization of Justicia spicigera. Food Chem. 218: 305–312 (2017).
Zou, TB, Xia, EQ, He, TP, Huang, MY, Jia, Q, Li, HW. Ultrasound-Assisted Extraction of Mangiferin from Mango (Mangifera indica L.) Leaves Using Response Surface Methodology. Molecules. 19: 1411–1421 (2014).
Zhu, Z, Guan, Q, Guo, Y, He, J, Liu, G, Li, S, Jaffrin, MY. Green ultrasound-assisted extraction of anthocyanin and phenolic compounds from purple sweet potato using response surface methodology. Int. Agrophys. 30(1): 113–122 (2016).
García-Márquez E, Román-Guerrero A, Pérez-Alonso C, Cruz-Sosa F, Jiménez-Alvarado R, Vernon-Carter EJ. Effect of solvent-temperature extraction conditions on the initial antioxidant activity and total phenolic content of muitle extracts and their decay upon storage at different pH. Rev. Mex. Ing. Quim. 11: 1–10 (2012).
Pérez-Jiménez J, Arranz S, Tabernero M, Díaz-Rubio ME, Serrano J, Goñi I, Saura-Calixto F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 41: 274–285 (2008).
Montreau F. Sur le dosage des composés phénoliques totaux dans les vins par la methode Folin-Ciocalteau. Connaiss Vigne Vin. 24: 397–404 (1972).
Hartzfeld PW, Forkner R, Hunter MD, Hagerman AE. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 50: 1785–1790 (2002).
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 26: 1231–1237 (1999).
Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 53: 4290–4302 (2005).
Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239: 70–76 (1996).
Aydar AY, Bagdathiglu N, Koseoglu O. Effect on olive oil extraction and optimization of ultrasound-assisted extraction of extra virgin olive oil by response surface methodology (RSM). Grasas y Aceites. 68(2): el89 (2017).
Kim HS, Lee AY, Jo JE, Moon BC, Chun JM, Choi G, Kim HK. Optimization of ultrasound-assisted extraction of quercitrin from Houttuynia cordata Thunb. using response surface methodology and UPLC analysis. Food Sci. Biotechnol. 23: 1–7 (2014).
Khan MK, Abert-Vian M, Fabiano-Tixier AS, Dangles O, Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 119: 851–858 (2010).
Ghitescu RE, Volf I, Carausu C, Buhlmann AM, Gilca IA, Popa VI. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 22: 535–541 (2015). 30.
Bashi DS, Mortazavi SA, Rezaei K, Rajaei A, Karimkhani MM. Optimization of ultrasound-assisted extraction of phenolic compounds from yarrow (Achillea beibrestinii) by response surface methodology. Food Sci. Biotechnol. 21: 1005–1011 (2012).
Pan Z, Qu W, Ma H, Atungulu GG, McHugh TH. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 18: 1249–1257 (2011).
Guerrero, S, López-Malo, A, Alzamora, SM. Effect of ultrasound on the survival of Saccharomyces cerevisiae: Influence of temperature, pH and amplitude. Innov. Food Sci. Emerg. Technol. 2, 31–39 (2001).
Guo L, Zhu WC, Liu YT, Wu JY, Zheng AQ, Liu YL. Response surface optimized extraction of flavonoids from mimenghua and its antioxidant activities in vitro. Food Sci. Biotechnol. 22: 1–8 (2013).
Michelon M, de Matos de Borba T, da Silva Rafael R, Burkert CAV, de Medeiros Burkert JF. Extraction of carotenoids from Phaffia rhodozyma: A comparison between different techniques of cell disruption. Food Sci. Biotechnol. 21: 1–8 (2012).
Nafar M, Emam-Djomeh Z, Yousefi S, Hashemi M. An optimization study on the ultrasonic treatments for Saccharomyces cerevisiae inactivation in red grape juice with maintaining critical quality attributes. J. Food Qual. 36: 269–281 (2013).
Yang RF, Huang PP, Qiu TQ. Ultrasound-enhanced subcritical water extraction of naphthoquinone pigments from purple gromwell (Lithospermum erythrorhizon) to higher yield and bioactivity. Food Sci. Biotechnol. 22: 671–676 (2013).
Sousa AD, Maia AIV, Rodrigues THS, Canuto KM, Ribeiro PRV, Pereira RDC. de Brito ES. Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF. Ind. Crops Prod. 79: 91–103 (2016). 39.
Sun J, Li X, Lin X, Mei Z, Li Y, Ding L, Bai W. Sonodegradation of cyanidin‐3‐glucosylrutinoside: degradation kinetic analysis and its impact on antioxidant capacity in vitro. J. Sci. Food Agric. 97: 1475–1481 (2017).
Acknowledgements
This research was financially supported by Tecnólogico Nacional de Mexico (Grant No. 5611.15-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Anaya-Esparza, L.M., Ramos-Aguirre, D., Zamora-Gasga, V.M. et al. Optimization of ultrasonic-assisted extraction of phenolic compounds from Justicia spicigera leaves. Food Sci Biotechnol 27, 1093–1102 (2018). https://doi.org/10.1007/s10068-018-0350-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0350-0