Abstract
It is known that flavonoids in sprouts were accumulated more under light irradiation than under dark. Light source affecting flavonoid accumulation in sprouts is still investigating. We evaluated the effects of light sources, including red, blue and fluorescent lights, on the flavonoid accumulation and antioxidant activity in common buckwheat sprouts. Experimental results showed that blue light significantly enhanced the contents of C-glycosylflavones, including orientin, vitexin and their isomers, and rutin and a rutin isomer. Sprouts grown under blue light exhibit also the highest total phenolics and total flavonoids as well as the highest antioxidant activities. It was found that isoorientin is the highest antioxidant flavonoid whereas numerous former studies suggested that rutin is a typical antioxidant compound in common buckwheat. These results indicated that blue light could be applied for enhancing not only the content of flavonoids but also antioxidant activity in common buckwheat sprouts.
Similar content being viewed by others
Introduction
Reactive oxygen species (ROS), considered harmful intermediates, are mainly produced in response to abiotic stress and respiration process. The production of excess ROS, originating from an imbalance between oxidants and antioxidants, causes DNA cleavage, protein oxidation, and lipid peroxidation in the body. All of these processes in turn induce pathophysiological events such as cancer and age-related diseases [1]. Hence, the balance between oxidation and the antioxidant defense system is crucial for maintaining a healthy biological system.
A low intake of vegetables has been increasingly reported to primarily induce a considerable number of ROS-related diseases [2]. Hence, consumers expect more physiological functions, such as antioxidants, besides the nutritional value from food products. Sprouts have been considered a popular health food because they contain a considerable amount of bioactive compounds such as minerals, amino acids, vitamins, and flavonoids [3]. As a result of these benefits, in recent years, sprouts have been widely consumed in the world. Common buckwheat (Fagopyrum esculentum Möench) sprouts, among the main edible buckwheat sprouts, have attracted considerable attention because of their abundant levels of flavonoids, including orientin, vitexin, rutin and their isomers [4]. Diets rich in buckwheat sprouts have been reported to prevent and/or reduce ROS-related diseases such as inflammation and neurological disorders [5, 6]. Among bioactive compounds in buckwheat sprouts, flavonoids, serving as antioxidants, play a key role in the prevention of above diseases [7].
The growth and development of plants are affected by various environmental conditions, such as water, temperature, light intensity, and light source. Environmental conditions, particularly light sources, including ultraviolet (UV), infrared and visible light, significantly affect flavonoid production during sprouting [8]. Use of light-emitting diodes (LEDs) has been reported to many advantages, such as controlling plant growth, providing higher energy conversion efficiency, longer life of light bulbs, lower thermal energy output, and selective wavelength [9]. In addition, recent studies proved that the application of LEDs to seedling plants gave beneficial effects related to the enhancement of bioactive compounds including β-carotene, vitamin C and tocopherol, and remarkable increase of antioxidant activity [10, 11]. Previous studies have reported that content of flavonoids in tartary buckwheat sprouts, one of main edible buckwheat sprouts, is markedly increased after LEDs irradiation [12,13,14]. However, the effects of LEDs on the composition of flavonoids and their concentrations in common buckwheat sprouts have rarely been reported [12]. Furthermore, recently, we reported the obvious analytical clue of existence of quercetin-3-O-robinobioside, a rutin isomer, in common buckwheat sprouts by HPLC–MS/MS and NMR analysis [15].
In this study, we investigated the effect of different types of light including red, blue and fluorescent on the accumulation of major flavonoids and the antioxidant activities in common buckwheat sprouts. Furthermore, the antioxidant activities of major individual flavonoids in the sprouts were investigated.
Materials and methods
Materials
Common buckwheat seeds were provided by Budnara plants company (Gwangju, Korea). Ascorbic acid, Folin–Ciocalteu’s phenol reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH), gallic acid, catechin, formic acid, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), orientin, isoorientin, vitexin, isovitexin, and rutin were products of Sigma-Aldrich Co. (St. Louis, MO, USA). 2,2′-Azobis(2-amidino-propane) dihydrochloride (AAPH) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All HPLC-grade solvents were used.
Growth condition and irradiation of common buckwheat sprouts
First, seeds were soaked in distilled water at room temperature for 4 h. Then, the imbibed seeds were sown into a dark growth chamber for 48 h to accelerate germination. The common buckwheat sprouts (CBS) were grown under different sources of light, including blue light (BL) and red light (RL) emitting diode lamps (P5II model supplying 3.3 V and 1 W per module, Seoul Semiconductor, Seoul, Korea), as well as fluorescent light (FL) lamps at a flux rate of 35 μmol/m2 s light intensity. The peak emissions of BL and RL were 460 and 625 nm, respectively. Sprouts grown under dark served as a non-light treatment control. Light condition, except for the control, was kept to 16 h of light supplement and 8 h of dark per day. Temperature was constantly kept to 25 °C. Sixty sprout individuals per each light treatment grown for 7 days were measured their shoot and root lengths. Seven-day-old sprouts were harvested and immediately dried in a 30 °C oven equipped with ventilation for 3 days. All samples were ground for flavonoid extraction.
Extraction of the flavonoids in common buckwheat sprouts
To extract flavonoids, a CBS powder (15 g) was mixed with 150 mL of absolute methanol and soaked for 48 h. The mixture was extracted using a homogenizer (Polytron PT 2100; Kinematica AG, Littau/Lucerne, 103 Switzerland) for 10 min. Then, the solvent was filtered through a Whatman #2 filter paper (Whatman International Limited, Kent, UK). The residues were re-extracted two times more using the same procedure described above. The filtrates were evaporated using a vacuum rotary evaporator (Eyela Co., Tokyo, Japan) with a 38 °C water bath. The extraction yields of CBS grown under BL, RL, FL and dark were approximately 44.8, 37.9, 38.4 and 32.4% based on dry weight, respectively. The extracts were stored at a −20 °C freezer before further subsequent experiments.
Total phenolic and flavonoid contents
Total phenolic content (TPC) was determined colorimetrically using Folin–Ciocalteu’s phenol reagent [16]. CBS extract (200 μL) concentrated to 1 mg/mL was mixed with 2.6 mL of deionized water and added to an additional aliquot (200 μL) of Folin–Ciocalteu’s phenol reagent. At 6 min, 2.0 mL of 7% (w/v) sodium carbonate solution was added to the reaction mixture. At 90 min, the mixture was vortexed and measured its absorbance at 750 nm using a spectrophotometer (S-1400, Scinco, Seoul, Korea). TPC was expressed as mg gallic acid equivalents (GAE)/g dry weight (DW).
Total flavonoid content (TFC) was measured according to a method [17] with some modifications. CBS extract (0.5 mL) concentrated to 1 mg/mL was mixed with 3.2 mL of deionized water, and added 0.15 mL of 5% sodium nitrate to the mixture. At 5 min, 0.15 mL of 10% (w/v) aluminum chloride was added, followed by the sequential addition of 1 mL of 1 M sodium hydroxide. Absorbance was measured immediately at 510 nm using the spectrophotometer described above. The results were expressed as mg quercetin equivalents (QE)/g DW.
HPLC analysis of flavonoids in CBS
The chromatographic separation of flavonoids present in the CBS extracts was carried out using a reversed-phase HPLC system (Shimadzu, Kyoto, Japan), equipped with an auto-sampler (SIL-20A, Shimadzu), a photodiode array detector (SPD-20A, Shimadzu), a binary pump (LC-20AD, Shimadzu), a vacuum degasser, and 5-C18-ace-EPS column (4.6 × 250 mm, 5.0 μm; Bischoff, Leonberg, Germany). The linear solvent gradient of the binary mobile phase was applied on the basis of our previous study [15]. The flow rate and injection volume were 0.8 mL/min and 20 μL, respectively. The detector was set at 350 nm for flavonoids in CBS extracts. Authentic flavonoids were used as standards for the calculation of the concentration of the individual flavonoids.
Antioxidant activity
DPPH free radical scavenging assay was conducted to determine the antioxidant activity. DPPH radical solution was prepared using 80% (v/v) aqueous methanol and adjusted its absorbance to 0.650 ± 0.020 at 517 nm. DPPH radical solution (2.95 mL) was added to each sprout extract (50 µL of 1 mg/mL) and allowed to stand at 23 °C in the dark for 30 min. The absorbance of solutions was subsequently measured at 517 nm.
ABTS radical decolorization assay was also evaluated for antioxidant activity of common buckwheat sprout extracts. The absorbance of the blue-green ABTS radical solution was adjusted to 0.650 ± 0.020 at 734 nm. The diluted samples (20 µL of 1 mg/mL) were added to the ABTS radical solution (980 µL) and incubated in a water bath at 37 °C for 10 min. The decrease of absorbance was measured at the end of time of 10 min. Antioxidant activities resulting both ABTS and DPPH free radical assays were expressed as mg vitamin C equivalents (VCE)/g DW.
Statistical analysis
All of the data are expressed as the means with standard deviation of three replicate determinations. One-way analysis of variance was applied to determine differences among means of treatments. Statistical analyses were conducted by Duncan’s multiple range test at the level of p < 0.05 using the SAS software (version 8.2, SAS Institute Inc., Cary, NC, USA). A Pearson correlation test was performed to evaluate the correlation among the variables and differences of p < 0.05 were considered significant.
Results and discussion
Effects of light sources on total phenolic and flavonoid contents
Sprouting is an effective technology for improving the contents of bioactive compounds and nutrients in seeds [18]. The nutritive value is highly dependent on the quality of seeds and the development stage of immature plants. Growing conditions, including light source, have been reported to possibly improve the nutrient contents in sprouts [19]. Flavonoids, representing the major active antioxidants in sprouts, possess a wide range of pharmaceutical properties including anti-tumor, anti-aging, and anti-inflammatory effects, as well as prevention of ROS-related diseases [7].
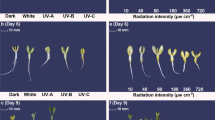
Figure 1A exhibits light influence on the shoot growth of buckwheat sprouts. Shoot lengths were longest in sprouts grown under dark condition. Red light grown sprouts exhibited longer shoot length than blue light grown sprouts. Fluorescent light grown sprouts ranked intermediate position between red and blue light grown sprouts. The results of this study are consistent with the results of Lim and Eom [20] that red light promotes shoot length of basil and blue light suppress the shoot length. Figure 1B shows root lengths of buckwheat sprouts grown under different light circumstances. Root length of buckwheat sprouts was longest in FL. Other treatments, including dark, RL, and BL, were shown no significant differences in root lengths. The dry weights of buckwheat sprouts among treatments were not significantly differed (data not shown). The effects of the different types of light on the TPC of CBS are shown in Fig. 2A. As compared to CBS grown under dark as a non-light treatment control, CBS exposed to light exhibited significant increase of TPC. The highest TPC (84.9 mg GAE/g DW) was observed in CBS grown under BL, followed by FL-exposed CBS (77.2 mg GAE/g DW) and RL-exposed CBS (64.1 mg GAE/g DW). Similarly, TFC of CBS exhibited considerable sensitivity to the types of light. All of the light treatments significantly enhanced the TFC, and the highest TFC was observed in BL-exposed CBS (Fig. 2B). The TFCs for all of the light-exposed CBS were approximately twofold higher than that of non-light treatment control. The TFCs of CBS exposed to BL, FL, and RL were 15.2, 13.4, and 11.9 mg QE/g DW, respectively.
Effect of light source on the concentration of flavonoids in sprouts
Qualitative analyses of major flavonoids (e.g., orientin, isoorientin, vitexin, isovitexin and rutin) in CBS have been previously reported using LC–MS/MS, and a flavonoid called quercetin-3-O-robinobioside (rutin isomer) has been additionally identified by using NMR [15]. In the present study, six flavonoids (orientin, vitexin, rutin, and their isomers) were detected (Fig. 3) and quantitatively analyzed by reversed-phase HPLC (Table 1). The contents of flavonoids in sprouts were markedly affected by light source. As compared to the dark condition, all of the tested lights led to a significant increase in flavonoids. The contents of the individual flavonoids in BL-exposed CBS were approximately 1.6–2.9-fold higher than that in the control. As compared to other lights, BL led to a significant increase of the individual flavonoids. The effects of RL treatment on the individual contents of flavonoids were similar to those of FL treatment. The highest flavonoid content in BL-exposed CBS was observed for rutin (5.5 mg/g DW), followed by isoorientin (5.1 mg/g DW), isovitexin (5.0 mg/g DW), vitexin (3.6 mg/g DW), quercetin-3-O-robinobioside (2.6 mg/g DW) and orientin (2.5 mg/g DW). Likewise, rutin and isoorientin were predominantly produced in CBS grown under RL and FL.
Lee et al. [12] have reported that light irradiation with BL and RL promotes the contents of flavonoids in buckwheat sprouts. The content of rutin in buckwheat sprouts irradiated with blue and red fluorescent lamps is significantly greater than that in sprouts grown in the dark [8]. The primary flavonoids in CBS grown under all of tested light types, as well as dark, were isoorientin, isovitexin and rutin (Table 1). C-Glycosylflavones such as isoorientin and isovitexin are the most common flavonoids in CBS and rutin is typically present in buckwheat seeds and sprouts [4]. According to Lee et al. [12], as compared to BL, RL promotes the production of flavonoids in CBS. Herein, however, a significant difference in the light source was observed. BL-treatment predominantly enhanced the contents of C-glycosylflavones and rutin in CBS (Table 1), indicating that flavonoid biosynthesis in buckwheat sprouts is reactive to both BL and RL. Previous studies have reported the effects of BL and RL on the accumulation of phenolic compounds in various plants, and conflicting results were frequently noted. Shiga et al. [21] have reported the effects of red, blue, and white light on TPC in sweet basil and the highest TPC is observed under white light treatment, followed by treatment with red and blue light. Nevertheless, the results obtained herein suggested that as compared to RL and FL, BL is more effective for the accumulation of flavonoids. There is a possible relationship between BL and the biosynthesis of phenylpropanoids by photosynthesis. Blue light photoreceptors, such as cryptochromes and phototropins, perceive specific wavelengths and regulate photomorphogenic responses and functional adaptations [22]. Cryptochromes photoreceptors induce the gene expression of flavonoid biosynthesis, e.g., chalcone synthase gene [23]. The results obtained may be explained by the biosynthesis of flavonoids through blue light-mediated receptors. However, the effect of light source on the mechanism of the changes in flavonoids contents is not well known yet. Short-wavelength lights, including blue and UV light, play important roles in the biosynthesis of flavonoids in seedling. Types of LEDs, exhibiting short-wavelength ranges, have been developed as light sources in plant production systems. Similar to that observed in this study, a previous study has reported the considerably increased contents of phenolic acids and flavonoids (rutin and kaempferol) in pea sprouts by treatment with BL (460 nm) as compared to RL (635 nm) and white fluorescent light [19]. As compared to green light (522.5 nm) and yellow light (594.5 nm), shorter-wavelength blue light (452.5 nm) significantly enhances the TFC in cucumber plant [24]. The synthesis of flavonoids in lettuce has been reportedly increased by BL treatment [25]. Wang et al. [26] have reported that the combination of BL and UV-B upregulates PAP1 and TT8, which are key regulatory genes of anthocyanin biosynthesis in plants. UV radiation, particularly the UV-B (>300 nm), effectively produces flavonoids in buckwheat sprouts [8]. Likewise, our results revealed that the spectrum of BL, close to the UV region, may promote increased production of flavonoids in CBS. Quercetin-3-O-robinobioside, consisting of quercetin with galactose-rhamnose, is structurally similar to rutin (quercetin with glucose-rhamnose). Although previous studies have investigated the effects of light source on the accumulation of major flavonoids in buckwheat sprouts [8, 12], marginal information is available about the relationship between light source and the production of quercetin-3-O-robinobioside in buckwheat sprouts. Quercetin-3-O-robinobioside tends to increase under all treated light-treatment (Table 1). The highest increase in the quercetin-3-O-robinobioside content was observed under BL. The content of quercetin-3-O-robinobioside in CBS grown under BL was 2.9 times that observed in CBS grown in the dark. Blue light may promote not only chalcone synthesis but also glycosylation of some flavonoid aglycones. Accordingly, the content of quercetin-3-O-robinobioside is possibly enhanced by BL-treatment. Previously, as compared to aglycone forms, quercetin glycosides have been reportedly absorbed more readily from small gut [27].
Antioxidant activities of sprouts grown under different types and their individual flavonoids
Figure 4A shows the effect of different light types on the antioxidant activity of CBS. Against the ABTS and DPPH radicals, antioxidant activity for CBS significantly increased under all of the light treatments as compared to the control. The highest antioxidant activity against the ABTS radical was found in BL-exposed CBS (37.7 mg VCE/g DW). No significant differences in the DPPH radical scavenging activity were observed among light-treated groups. A wide range of antioxidant activities was observed in major flavonoids from CBS (Fig. 4B). Isoorientin exhibited the highest DPPH radical scavenging activity, with an antioxidant activity of 1727.0 mg VCE/g. In addition, rutin (799.9 mg VCE/g) and orientin (775.9 mg VCE/g) exhibited remarkably decreased DPPH radical. Vitexin and isovitexin showed extremely weak DPPH radical scavenging activity with a value of 12.47 and 16.04 mg VCE/g, respectively. In addition, the highest antioxidant against the ABTS assay was observed in isoorientin. The overall antioxidant activity of individual flavonoids against ABTS radical decreased in the following order: isoorientin (1728.0 mg VCE/g) > rutin (941.1 mg VCE/g) > orientin (766.2 mg VCE/g) > isovitexin (675.8 mg VCE/g) > vitexin (243.4 mg VCE/g).
Antioxidant activities of common buckwheat sprouts grown under different types of light sources (A) and their individual flavonoids (B). Antioxidant activities were evaluated using ABTS and DPPH radical scavenging assays. Each data is the mean ± SD of three replicates. Different letters above bars of the same color indicate significant differences at p < 0.05 by Duncan’s multiple range test
A correlation analysis was performed with the results obtained by different methods (Table 2). High correlations were observed between TPC and TFC (r 2 = 0.937, p < 0.01), and TPC and ABTS assay (r 2 = 0.950, p < 0.01). In addition, TFC and ABTS assay positively correlated with each other (p < 0.05). The lowest correlations were observed between TFC and DPPH assay (r 2 = 0.796).
ABTS and DPPH assays have been widely used to determine the antioxidant activity of plant extracts based on the ability of an electron transfer antioxidant to reduce oxidants. The antioxidant activities of extracts are considerably affected by the extract composition and experimental conditions [28]. There is no single assay for measuring the overall antioxidant activities, and more than one type of antioxidant activity assay needs to be performed to examine the different modes of antioxidant action [29]. Light source significantly affected the antioxidant activities of buckwheat sprouts (Fig. 4A). The highest ABTS radical scavenging activity of CBS was observed under BL treatment, possibly related to the highest TPC and TFC observed (Fig. 2). Significant correlations were observed between TPC (r 2 = 0.950, p < 0.01), and TFC (r 2 = 0.876, p < 0.05) and ABTS in Table 2. Additionally, a positive correlation between TPC (r 2 = 0.857), and TFC (r 2 = 0.796) and DPPH assay (r 2 = 0.857) was noted. However, no significant differences in the DPPH radical scavenging activity, as well as a slight increase in the antioxidant activity against the ABTS radicals, were observed between BL treatment and other light treatments (Fig. 4A). Qian et al. [11] have reported that concentration of vitamin C in the seedlings of Chinese kale sprouts are significantly greater under RL and FL as compared with those of sprouts under BL. β-Carotene content in pea seedlings was significantly higher under RL than that under BL [10]. Our results obtained may be explained by the fact that besides flavonoids, other substances in RL- and FL-treated sprouts possibly contribute to antioxidant activity.
Among the five individual flavonoids, isoorientin exhibited the highest radical scavenging activity using ABTS and DPPH radicals, followed by rutin and orientin. Phenolic compounds including flavonoids and phenolic acids have been demonstrated to contribute more to antioxidant activity than other antioxidants [30]. Rutin is well known for its beneficial biological effects as antioxidant. Besides rutin, orientin and isoorientin were produced in high concentrations under BL treatment, and they exhibited high antioxidant activities. On the other hand, vitexin and isovitexin showed relatively (ABTS assay) or extremely (DPPH assay) weak radical scavenging activity. Vitexin and isovitexin contain a single B-ring OH group and two A-ring OH groups, while orientin and isoorientin contain two B-ring OH groups (ortho-dihydroxy structure) and two A-ring OH groups. Previously, an apparent positive correlation between the number of OH groups in the B-ring and antioxidant activity has been reported [31]. The OH group in the A-ring exhibits marginal correlation with the radical scavenging activity. Moreover, the B-ring with an ortho-dihydroxy structure confers high stability to the flavonoid phenoxyl radicals [32]. Unfortunately, the antioxidant activity of quercetin-3-O-robinobioside could not be estimated. High purity quercetin-3-O-robinobioside was not successfully isolated from CBS because of the difficulty associated with its separation from rutin, related to their similar polarities. Hence, additional studies are needed to estimate the health benefits and biological activities of quercetin-3-O-robinobioside.
In conclusion, the results revealed that BL is effective and suitable for growing buckwheat sprouts, with increased TPC, TFC and antioxidant capacity using ABTS radical. Furthermore, BL considerably enhanced the contents of orientin, isoorientin, vitexin, isovitexin, and rutin as well as accumulation of quercetin-3-O-robinobioside in CBS. Isoorientin, one of major flavonoids in CBS, exhibited the most powerful antioxidant activity among the tested individual flavonoids. Thus, the results indicated that BL can be applied to enhance the content of flavonoids in buckwheat sprouts. However, no significant differences in the DPPH radical scavenging activity were observed among light-treated groups. There is a possibility that besides flavonoids, other substances produced in RL- and FL-treated sprouts may contribute to antioxidant activity. Further research is required to explain the other non-ignorable antioxidants in CBS grown under different light sources.
References
Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 3: 1129–1134 (2002)
Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer 18: 1–29 (1992)
Kim S-L, Kim S-K, Park C-H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int. 37: 319–327 (2004)
Kim S-J, Zaidul ISM, Suzuki T, Mukasa Y, Hashimoto N, Takigawa S, Noda T, Matsuura-Endo C, Yamauchi H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 110: 814–820 (2008)
Gulpinar AR, Orhan IE, Kan A, Senol FS, Celik SA, Kartal M. Estimation of in vitro neuroprotective properties and quantification of rutin and fatty acids in buckwheat (Fagopyrum esculentum Moench) cultivated in Turkey. Food Res. Int. 46: 536–543 (2012)
Ishii S, Katsumura T, Shiozuka C, Ooyauchi K, Kawasaki K, Takigawa S, Fukushima T, Tokuji Y, Kinoshita M, Ohnishi M. Anti-inflammatory effect of buckwheat sprouts in lipopolysaccharide-activated human colon cancer cells and mice. Biosci. Biotechnol. Biochem. 72: 3148–3157 (2008)
Xiao J, Capanoglu E, Jassbi AR, Miron A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 56: S29–S45 (2016)
Tsurunaga Y, Takahashi T, Katsube T, Kudo A, Kuramitsu O, Ishiwata M, Matsumoto S. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem. 141: 552–556 (2013)
Schuerger AC, Brown CS, Stryjewski EC. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 79: 273–282 (1997)
Wu M-C, Hou C-Y, Jiang C-M, Wang Y-T, Wang C-Y, Chen H-H, Chang H-M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 101: 1753–1758 (2007)
Qian H, Liu T, Deng M, Miao H, Cai C, Shen W, Wang Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 196: 1232–1238 (2016)
Lee S-W, Seo JM, Lee M-K, Chun J-H, Antonisamy P, Arasu MV, Suzuki T, Al-Dhabi NA, Kim S-J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crops Prod. 54: 320–326 (2014)
Thwe AA, Kim YB, Li X, Seo JM, Kim S-J, Suzuki T, Chung S-O, Park SU. Effects of light-emitting diodes on expression of phenylpropanoid biosynthetic genes and accumulation of phenylpropanoids in Fagopyrum tataricum sprouts. J. Agr. Food Chem. 62: 4839–4845 (2014)
Seo J-M, Arasu MV, Kim Y-B, Park SU, Kim S-J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 177: 204–213 (2015)
Nam TG, Lee SM, Park J-H, Kim D-O, Baek N-i, Eom SH. Flavonoid analysis of buckwheat sprouts. Food Chem. 170: 97–101 (2015)
Singleton VL, Rossi JA, Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16: 144–158 (1965)
Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64: 555–559 (1999)
Tang D, Dong Y, Guo N, Li L, Ren H. Metabolomic analysis of the polyphenols in germinating mung beans (Vigna radiata) seeds and sprouts. J. Sci. Food. Agric. 94: 1639–1647 (2014)
Liu H, Chen Y, Hu T, Zhang S, Zhang Y, Zhao T, Yu H, Kang Y. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 25: 459–465 (2016)
Lim YJ, Eom SH. Effects of different light types on root formation of Ocimum basilicum L. cuttings. Sci. Hort. 164: 552–555 (2013)
Shiga T, Shoji K, Shimada H, Hashida S-n, Goto F, Yoshihara T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 26: 255–259 (2009)
Takemiya A, Inoue S, Doi M, Kinoshita T, Shimazaki K. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell. 17: 1120–1127 (2005)
Wade HK, Bibikova TN, Valentine WJ, Jenkins GI. Interactions within a network of phytochrome, cryptochrome and UV‐B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J. 25: 675–685 (2001)
Wang H, Jiang YP, Yu HJ, Xia XJ, Shi K, Zhou YH, Yu JQ. Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur. J. Plant Pathol. 127: 125–135 (2010)
Taulavuori K, Hyöky V, Oksanen J, Taulavuori E, Julkunen-Tiitto R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 121: 145–150 (2016)
Wang J, Wang Y, Chen B, Kawabata S, Li Y. Comparative transcriptome analysis revealed distinct gene set expression associated with anthocyanin biosynthesis in response to short-wavelength light in turnip. Acta Physiol. Plant. 38: 1–12 (2016)
Hollman PC, van Trijp JM, Buysman MN, Mengelers MJ, de Vries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 418: 152–156 (1997)
Li H-B, Wong C-C, Cheng K-W, Chen F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Technol. 41: 385–390 (2008)
Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J. Agr. Food Chem. 53: 1841–1856 (2005)
Kim D-O, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agr. Food Chem. 50: 3713–3717 (2002)
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 13: 572–584 (2002)
Mora A, Paya M, Rios J, Alcaraz M. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem. Pharmacol. 40: 793–797 (1990)
Acknowledgements
This study was supported by a grant from the Individual Basic Science and Engineering Research Program, National Research Foundation of Korea, Republic of Korea (Grant No. NRF-2014R1A1A2058838).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nam, T.G., Kim, DO. & Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci Biotechnol 27, 169–176 (2018). https://doi.org/10.1007/s10068-017-0204-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0204-1