Abstract
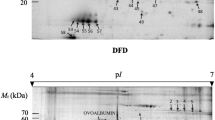
A phosphoproteomic profile of myofibrillar and sarcoplasmic proteins of muscle in response to salting was investigated. Myofibrillar and sarcoplasmic proteins extracted from salted meat with 0, 1, 2, 3, 4, and 5% salt for 0, 2, 4, 6, 8, and 16 h were analyzed by SDS-PAGE electrophoresis and fluorescence staining. The global phosphorylation of myofibrillar proteins in salted meat was lower than that in control muscle at 16 h of salting (p<0.05), and the global phosphorylation of myofibrillar proteins in 3% salt-treated group at 16 h was the lowest. However, salting showed no significant effect on phosphorylation of sarcoplasmic proteins. Four categories of phosphorylated protein were identified by LC-MS/MS, involved in stress response (heat shock protein), glycometabolism (glycogen phosphorylase, glyceraldehyde-3-phosphate dehydrogenase), oxidation or reduction (superoxide dismutase), and others (myoglobin), the phosphorylation of which was affected by salting. Thus, salting may influence meat quality through protein phosphorylation, which regulates protein degradation and glycolysis.
Similar content being viewed by others
References
Barat JM, Baigts D, Aliño M, Fernández FJ, Pérez-García VM. Kinetics studies during NaCl and KCl pork meat brining. J. Food Eng. 106: 102–110 (2011)
Binkerd EF, Kolari OE. The history and use of nitrate and nitrite in the curing of meat. Food Cosmet. Toxicol. 13: 655–716 (1975)
Olesen PT, Meyer AS, Stahnke LH. Generation of flavour compounds in fermented sausages-the influence of curing ingredients, Staphylococcus starter culture and ripening time. Meat Sci. 66: 675–687 (2004)
Comaposada J, Gou P, Arnau J. The effect of sodium chloride content and temperature on pork meat isotherms. Meat Sci. 55: 291–295 (2000)
Thorarinsdottir KA, Arason S, Sigurgisladottir S, Gunnlaugsson VN, Johannsdottir J, Tornberg E. The effects of salt-curing and salting procedures on the microstructure of cod (Gadus morhua) muscle. Food Chem. 126: 109–115 (2011)
Bombrun L, Gatellier P, Carlier M, Kondjoyan A. The effects of low salt concentrations on the mechanism of adhesion between two pieces of pork semimembranosus muscle following tumbling and cooking. Meat Sci. 96: 5–13 (2014)
Cherroud S, Cachaldora A, Fonseca S, Laglaoui A, Carballo J, Franco I. Microbiological and physicochemical characterization of dry-cured Halal goat meat. Effect of salting time and addition of olive oil and paprika covering. Meat Sci. 98: 129–134 (2014)
Han G, Ye M, Zou H. Development of phosphopeptide enrichment techniques for phosphoproteome analysis. Analyst 133: 1128–1138 (2008)
Chen L, Li X, Ni N, Liu Y, Chen L, Wang Z, Shen QW, Zhang D. Phosphorylation of myofibrillar proteins in post-mortem ovine muscle with different tenderness. J. Sci. Food Agr. 96: 1474–1483 (2015)
Nishi H, Shaytan A, Panchenko AR. Physicochemical mechanisms of protein regulation by phosphorylation. Front. Genet. 5: 270 (2014)
Di Lisa F, De Tullio R, Salamino F, Barbato R, Melloni E, Siliprandi N, Schiaffino S, Pontremoli S. Specific degradation of troponin T and I by mu-calpain and its modulation by substrate phosphorylation. Biochem. J. 308: 57–61 (1995)
Toyo-oka T. Phosphorylation with cyclic adenosine 3:5 monophosphatedependent protein kinase renders bovine cardiac troponin sensitive to the degradation by calcium-activated neutral protease. Biochem. Bioph. Res. Co. 107: 44–50 (1982)
Huang H, Larsen MR, Lametsch R. Changes in phosphorylation of myofibrillar proteins during postmortem development of porcine muscle. Food Chem. 134: 1999–2006 (2012)
Huang H, Larsen MR, Karlsson AH, Pomponio L, Costa LN, Lametsch R. Gelbased phosphoproteomics analysis of sarcoplasmic proteins in postmortem porcine muscle with pH decline rate and time differences. Proteomics 11: 4063–4076 (2011)
Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT. Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. P. Natl. Acad. Sci. USA 102: 17519–17524 (2005)
Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch. Biochem. Biophys. 510: 120–128 (2011)
Li C, Zhou G, Xu X, Lundström K, Karlsson A, Lametsch R. Phosphoproteome analysis of sarcoplasmic and myofibrillar proteins in bovine longissimus muscle in response to postmortem electrical stimulation. Food Chem. 175: 197–202 (2015)
Hu Y, Guo S, Li X, Ren X. Comparative analysis of salt-responsive phosphoproteins in maize leaves using Ti(4+)—IMAC enrichment and ESI-Q-TOF MS. Electrophoresis 34: 485–492 (2013)
Zeniya M, Sohara E, Kita S, Iwamoto T, Susa K, Mori T, Oi K, Chiga M, Takahashi D, Yang SS, Lin SH, Rai T, Sasaki S, Uchida S. Dietary salt intake regulates WNK3-SPAK-NKCC1 phosphorylation cascade in mouse aorta through angiotensin II. Hypertension 62: 872–878 (2013)
Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 74: 1403–1409 (2008)
Lametsch R, Kristensen L, Larsen MR, Therkildsen M, Oksbjerg N, Ertbjerg P. Changes in the muscle proteome after compensatory growth in pigs. J. Anim. Sci. 84: 918–924 (2006)
Schulenberg B, Aggeler R, Beechem JM, Capaldi RA, Patton WF. Analysis of steady-state protein phosphorylation in mitochondria using a novel fluorescent phosphosensor dye. J. Biol. Chem. 278: 27251–27255 (2003)
Mora-Gallego H, Guardia MD, Serra X, Gou P, Arnau J. Sensory characterisation and consumer acceptability of potassium chloride and sunflower oil addition in small-caliber non-acid fermented sausages with a reduced content of sodium chloride and fat. Meat Sci. 112: 9–15 (2015)
Sheard PR, Tali A. Injection of salt, tripolyphosphate and bicarbonate marinade solutions to improve the yield and tenderness of cooked pork loin. Meat Sci. 68: 305–311 (2004)
D’Alessandro A, Rinalducci S, Marrocco C, Zolla V, Napolitano F, Zolla L. Love me tender: An omics window on the bovine meat tenderness network. J. Proteomics 75: 4360–4380 (2012)
Zhang Z, Lawrence J, Stracher A. Phosphorylation of platelet actin binding protein protects against proteolysis by calcium dependent sulfhydryl protease. Biochem. Bioph. Res. Co. 151: 355–360 (1988)
Silverman-Gavrila LB, Lu TZ, Prashad RC, Nejatbakhsh N, Charlton MP, Feng ZP. Neural phosphoproteomics of a chronic hypoxia model—Lymnaea stagnalis. Neuroscience 161: 621–634 (2009)
Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 68: 513–519 (2015)
Sale EM, White MF, Kahn CR. Phosphorylation of glycolytic and gluconeogenic enzymes by the insulin receptor kinase. J. Cell. Biochem. 33: 15–26 (1987)
Muller MS, Pedersen SE, Walls AB, Waagepetersen HS, Bak LK. Isoformselective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63: 154–162 (2015)
Bell RAV, Storey KB. P06: Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase from a hibernating mammal: Insight into coldadaptation and structural diversity of a housekeeping enzyme. Cryobiology 69: 196–197 (2014)
Gonzalez B, Manso R. Induction, modification and accumulation of HSP70s in the rat liver after acute exercise: Early and late responses. J. Physiol.-London 556: 369–385 (2004)
Melling CW, Thorp DB, Milne KJ, Noble EG. Myocardial Hsp70 phosphorylation and PKC-mediated cardioprotection following exercise. Cell Stress Chaperon. 14: 141–150 (2009)
Carvalho ME, Gasparin G, Poleti MD, Rosa AF, Balieiro JC, Labate CA, Nassu RT, Tullio RR, Regitano LC, Mourao GB, Coutinho LL. Heat shock and structural proteins associated with meat tenderness in Nellore beef cattle, a Bos indicus breed. Meat Sci. 96: 1318–1324 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Wang, Z., Li, Z. et al. Phosphoproteomic profiling of myofibrillar and sarcoplasmic proteins of muscle in response to salting. Food Sci Biotechnol 25, 993–1001 (2016). https://doi.org/10.1007/s10068-016-0161-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-016-0161-0