Abstract
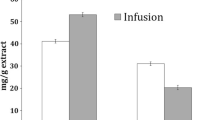
Traditionally used medicinal plants contain a wide range of polyphenolic compounds that act as powerful antioxidants. The content of phenolic compounds in the infusions and decoctions of chamomile (Matricaria chamomilla L.) and St. John’s wort (Hypericum perforatum), which are traditionally used medicinal herbs, was evaluated via liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. To obtain relevant antioxidant/reducing capacity of the prepared extracts, cupric reducing antioxidant capacity and Folin-Ciocalteu assay were applied. Rutin and apigenin were the major flavonoids in the aqueous extract of chamomile, whereas the predominant phenolic compounds of St. John’s wort water extracts were rutin and catechin followed by chlorogenic acid. A longer time of infusion and decoction of St. John’s wort herb significantly affected the rutin content. The increase of extraction time had very little impact on the antioxidant activities for chamomile but considerably higher impact on those for St. John’s wort.
Similar content being viewed by others
References
McKav DL, Blumberg JB. A review of the bioactivity and potential benefits of chamomile tea (Matricaria recuita L.). Phytother. Res. 20: 519–530 (2006)
Chan EWC, Lim YY, Chong KL, Tan JBL, Wong SK. Antioxidant properties of tropical and temperate herbal teas. J. Food Compos. Anal. 23: 185–189 (2012)
Euromonitor International. Herbal Supplements and Remedies Marked Trends. Available from: http://www.euromonitor.com/herbal-traditionalproducts. Accessed Nov. 11, 2015
Galleano M, Verstraeten SV, Oleiza PI, Firaga CE. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 401: 23–30 (2010)
Santhakumar AB, Bulmer AC, Singh J. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet. 27: 1–21 (2013)
Dastmalchi K, Dorman HD, Oinonen PP, Darvis Y, Laakso I, Hiltuen R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT-Food Sci. Technol. 41: 391–400 (2008)
Komes D, Horžic D, Belšæak A, Ganiæ KK, Vukiæ I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 43: 167–176 (2010)
Chandini SK, Rao JL, Subramamian R. Influence of extraction conditions on polyphenols content and cream constituents in black tea extracts. Int. J. Food Sci. Tech. 46: 879–886 (2011)
Biesaga M, Pyrzynska K. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 136: 46–54 (2013)
Sentkowska A, Biesaga M, Pyrzynska K. Effects of the operation parameters on HILIC separation of flavonoids on zwitterionic column. Talanta 115: 284–290 (2013)
Sentkowska A, Biesaga M, Pyrzynska K. Polyphenolic composition and antioxidative properties of lemon balm (Melissa officinalis L.) extract affected by different brewing processes. Int. J. Food Prop. 18: 2009–2014 (2015)
Apak R, Güçlü K, Özyürek M, Karademir S E, Erçag E. Mechanism and antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 160: 413–419 (2008)
Dias MM, Machado NF, Marques MPM. Dietary chromoes as antioxidant agents–the structural variable. Food Funct. 2: 595–602 (2012)
Sentkowska A, Biesaga M, Pyrzynska K. Retention study of flavonoids under different chromatographic modes. J. Chromatogr. Sci. 54: 516–522 (2016)
Gawlik-Dziki U, Swieca M, Sulkowski M, Dziki D, Baraniak B, Czyz J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts-in vitro study. Food Chem. Toxicol. 5: 154–160 (2013)
Azevedo MI, Pereira MF, Nogueira RB, Rolim FE, Brito GAC, Wong DV, Lima-Júnior RCP, Ribeiro RA, Vale ML. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 9: 33–36 (2013)
Li W, Sun Y, Liang W, Fitzlof JF, van Breemen RB. Identification of caffeic acid derivatives in Actea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/ tandem mass spectrometry. Rapid Commun. Mass Sp. 17: 978–982 (2003)
Xia YQ, Miller JD, Bakhtiar R, Fitzloff JF, van Breemen RB. Use of a quadrupole linear ion trap mass spectrometer in metabolite identification and bioanalysis. Rapid Commun. Mass Sp. 17: 1137–1145 (2003)
Novakova L, Vildova A, Mateus JP, Goncalves, T, Solich P. Development and application of UHPLC–MS/MS method for the determination of phenolic compounds in Chamomile flowers and Chamomile tea extracts. Talanta 82: 1271–1280 (2010)
Raal A, Orav A, Pusa T, Valner C, Malmiste B, Arak E. Content of essential oil, terpenoids and polyphenols in commercial chamomile (Chamomilia recrutita L. Rauschert) teas from different countries. Food Chem. 131: 632–638 (2012)
Avula B, Wang, YH, Wang M, Avonto C, Zhao J, Smillie TJ, Rua D, Khan IA. Quantitative determination of phenolic compounds by UHPLC-UV-MS and the use of partial least-square discriminate analysis to differentiate chemo-types of Chamomile/Chrysanthemum flower heads. J. Pharmaceut. Biomed. 8: 278–288. (2014)
Azmir J, Zaidul ISM, Rahman MM, Shari KM, Mohamed A, Saherna F, Jahurul MHA, Ghafoor K, Norulaini, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 117: 426–436 (2013)
Guimarães R, Barros L, Duenas M, Calhelha RC, Carvalho AM, Santos-Buelga C, Queiroz MJRP, Ferreira ICFR. Infusion and decoction of wild German chamomile. Bioactivity and characterization of organic acids and phenolic compounds. Food Chem. 136: 947–954 (2013)
Murakami M, Yamaguchi T, Takamura H, Matoba, T. Effects of thermal treatment on radical-scavenging activity of single and mixed polyphenolic compounds. Food Chem. Toxicol. 69: 7–10 (2004)
Buchner N, Krumbein A, Rohn S, Kroh LW. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Sp. 20: 3229–3235 (2006)
Dadakova E, Vrchotova N, Chmelova S, Sera B. The stability of rutin and chlorogenic acid during the processing of black elder (Sambuscus Nigra) in fluorescence. Acta Aliment. Hung 40: 327–334 (2011)
Ioannou I, Hafsa I, Hamdi S, Charnonnel C, Ghoul M. Review of the effects of food processing and formulation on flavonol and anthocyanins behavior. J. Food Eng. 111: 208–217 (2012)
Harbourne N, Marete E, Jacquier JC, O’Riordan D. Stability of phytochemicals as sources of anti-inflammatory nutriceuticals in beverages -A review. Food Res. Int. 50: 480–486 (2013)
Gonthier MP, Verny AM, Besson C, Remesy C, Scalbert A. Chlorogenic acid bioavailability largely depends on its metabolizm by the gut microflora in rats. J. Nutr. 133: 1853–1859 (2003)
Narita Y, Inouye K. Degradation kinetics of chlorogenic acid at arious pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions. J. Agr. Food Chem. 61: 966–972 (2013)
Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçl K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 85: 957–998 (2013)
Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB. Thorough study of reactivity of various compound classes toward Folin–Ciocalteu reagent, J. Agr. Food Chem. 58: 8139–8144 (2010)
Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 113: 1226–1233 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sentkowska, A., Biesaga, M. & Pyrzynska, K. Effects of brewing process on phenolic compounds and antioxidant activity of herbs. Food Sci Biotechnol 25, 965–970 (2016). https://doi.org/10.1007/s10068-016-0157-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-016-0157-9