Abstract
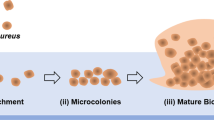
This study investigated biofilm formation, cell surface hydrophobicity, colony spreading, and slime production for 112 Staphylococcus aureus strains isolated from various sources (leaf vegetables, pea leaf, perilla leaf, Kim-bab, person, and animal). When biofilm formation was classified by origin, S. aureus isolated from animal origin showed a significantly higher level of biofilm formation than others (p≤0.05). When S. aureus groups with different levels of biofilm formation (very strong, strong, moderate, and weak) were evaluated for the correlation with cell surface properties, there was a positive correlation between biofilm formation and hydrophobicity (r=0.926). Biofilm formation and colony spreading on tryptic soy broth (without dextrose) also showed positive correlation (r=0.863). In contrast, biofilm formation and slime production were negatively correlated (r=−0.973). Based on these results, the biofilm forming ability of S. aureus differs depending on their origin and might be affected by cell surface properties such as cell surface hydrophobicity.
Similar content being viewed by others
References
Kaito C, Sekimizu K. Colony spreading in Staphylococcus aureus. J. Bacteriol. 189: 2553–2557 (2007)
Jamalia H, Paydar M, Radmehr B, Ismail S, Dadrasnia A. Prevalence and antimicrobial resistance of Staphylococcus aureus isolated from raw milk and dairy products. Food Control 54: 383–388 (2015)
Enright MC. The evolution of resistant pathogen-the case of MRSA. Curr. Opin. Pharmacol. 3: 474–479 (2003)
Gorman R, Bloomfield S, Adley CC. A study of cross-contamination of foodborne pathogens in the domestic kitchen in the Republic of Ireland. Int. J. Food Microbiol. 76: 143–150 (2002)
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu. Rev. Microbiol. 49: 711–745 (1995)
Xu H, Lee HY, Ahn J. Characteristics of biofilm formation by selected foodborne pathogens. J. Food Safety 31: 91–97 (2011)
Pagedar A, Singh J, Batish VK. Surface hydrophobicity, nutritional contents affect Staphylococcus aureus biofilms and temperature influences its survival in preformed biofilms. J. Basic Microb. 50: S98–S106 (2010)
Götz F. Staphylococcus and biofilms. Mol. Microbiol. 43: 1367–1378 (2002)
Vatsos IN, Thompson KD, Adams A. Adhesion of the fish pathogen Flavobacterium psychrophilum to unfertilized eggs of rainbow trout (Oncorhynchus mykiss) and n-hexadecane. Lett. Appl. Microbiol. 33: 178–182 (2001)
Sibbald MJJB, Ziebandt AK, Engelmann S, Hecker M, de Jong A, Harmsen HJM, Raangs GC, Stokroos I, Arends JP, Dubois JYF, van Dijl JM. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol. Mol. Biol. R. 70: 755–788 (2006)
Gallardo-Moreno AM, González-Martín ML, Pérez-Giraldo C, Bruque JM, Gómez-García AC. Serum as a factor influencing adhesion of Enterococcus faecalis to glass and silicone. Appl. Environ. Microb. 68: 5784–5787 (2002)
Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, Lyons CR, Byrd TF. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 152: 1581–1590 (2006)
Deighton MA, Capstick J, Borland R. A study of phenotypic variation of Staphylococcus epidermidis using Congo red agar. Epidemiol. Infect. 109: 423–432 (1992)
Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slimeproducing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37: 318–326 (1982)
Arciola CR, Campoccia D, Montanaro L. Detection of biofilm-forming strains of Staphylococcus epidermidis and S. aureus. Expert Rev. Mol. Diagn. 2: 478–484 (2002)
Djordjevic D, Wiedmann M, Mclandsborough LA. Microtitier plates assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microb. 68: 2950–2958 (2002)
Goulter RM, Gentle IR, Dykes GA. Characterisation of curli production, cell surface hydrophobicity, autoaggregation and attachment behavior of Escherichia coli O157. Curr. Microbiol. 61: 157–162 (2010)
Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 42: 872–874 (1989)
Oliveira A, Cunha Mde L. Comparison of methods for the detection of biofilm production in coagulase-negative staphylococci. BMC Res. Notes 3: 260 (2010)
Stepanoviæ S, Vukoviæ D, Hola V, Di Bonaventura G, Djukiæ S, Cirkoviæ I, Ruzicka F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115: 891–899 (2007)
Cha JO, Park YK, Lee YS, Chung GT. In vitro biofilm formation and bacterial activities of methicillin-resistant Staphylococcus aureus clones prevalent in Korea. Diagn. Micr. Infec. Dis. 70: 112–118 (2011)
Takahashi H, Miya S, Igarashi K, Suda T, Kuramoto S, Kimura B. Biofilm formation ability of Listeria monocytogenes isolates from raw ready-to-eat seafood. J. Food Protect. 72: 1476–1480 (2009)
Nilsson RE, Ross T, Bowman JP. Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int. J. Food Microbiol. 150: 14–24 (2011)
Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72: 3658–3663 (2004)
Takahashi H, Suda T, Tanaka Y, Kimura B. Cellular hydrophobicity of Listeria monocytogenes involves initial attachment and biofilm formation on the surface of polyvinyl chloride. Lett. Appl. Microbiol. 50: 618–625 (2010)
Pasmore M, Todd P, Smith S, Baker D, Silverstein J, Coons D, Bowman CN. Effects of ultrafiltration membrane surface properties on Pseudomonas aeruginosa biofilm initiation for the purpose of reducing biofouling. J. Membrane Sci. 30: 15–32 (2001)
Ukuku DO, Fett WF. Relationship of cell surface charge and hydrophobicity to strength of attachment of bacteria to cantaloupe rind. J. Food Protect. 65: 1093–1099 (2002)
Auger S, Ramarao N, Faille C, Fouet A, Aymerich S, Gohar M. Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl. Environ. Microb. 75: 6616–6618 (2009)
Flint SH, Brooks JD, Bremer PJ. The influence of cell surface properties of thermophilic streptococci on attachment to stainless steel. J. Appl. Microbiol. 93: 508–517 (1997)
O’Toole GA. Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30: 295–304 (1998)
Agustí G, Astola O, Rodríguez-Güell E, Julián E, Luquin M. Surface spreading motility shown by a group of phylogenetically related, rapidly growing pigmented mycobacteria suggests that motility is a common property of mycobacterial species but is restricted to smooth colonies. J. Bacteriol. 190: 6894–6902 (2008)
Ueda T, Kaico C, Omae Y, Sekimizu K. Sugar-responsive gene expression and the agr system are required for colony spreading in Staphylococcus aureus. Microb. Pathogenesis 51: 178–185 (2011)
Tojo M, Yamashita N, Goldmann DA, Pier GB. Isolation and characterization of a capsular polysaccharide adhesion from Staphylococcus epidermidis. J. Infect. Dis. 157: 713–722 (1988)
Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes slime production in a collection of staphylococcal strains from catheter-associated infections. J. Clin. Microbiol. 39: 2151–2156 (2001)
Oliveria M, Bexiga R, Nunes SF, Carneiro C, Cavaco LM, Bernardo F, Vilela CL. Biofilm-forming ability profiling of Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet. Microbiol. 118: 133–140 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, BR., Bae, YM., Hwang, JH. et al. Biofilm formation and cell surface properties of Staphylococcus aureus isolates from various sources. Food Sci Biotechnol 25, 643–648 (2016). https://doi.org/10.1007/s10068-016-0090-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-016-0090-y