Abstract
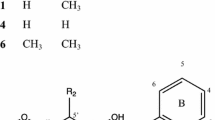
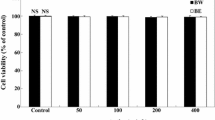
In this study, we investigated the anti-adipogenic effects of Eleutherococcus senticosus and its active compounds in vitro to examine new functions. We first analyzed the active compounds in E. senticosus growing in Korea using HPLC and found that the concentration of eleutheroside B and E was higher in stems and roots than in other plant parts. There were no significant (p<0.05) differences in eleutheroside concentration between plant ages. Anti-adipogenic effects of E. senticosus on lipid accumulation in 3T3-L1 cells were examined. Extracts of stems and roots more effectively inhibited lipid accumulation in 3T3-L1 cells than extracts of other plant parts. Eleutheroside E was responsible for the pharmacological anti-adipogenic effects via regulation of the mTOR pathway. This is the first report of an anti-adipogenic effect of E. senticosus and the active compound eleutheroside E.
Similar content being viewed by others
References
Brekhman II, Dardymov IV. New substances of plant origin which increase nonspecific resistance. Ann. Rev. Pharmacol. 9: 419–430 (1969)
Davydov M, Krikorian AD. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. J. Ethnopharmacol. 72: 345–393 (2000)
Huang L, Zhao H, Huang B, Zheng C. Peng W. Qin L. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Pharmazie 66: 83–97 (2011)
Zaluski D, Smolarz HD, Szpilewska M. Eleutherosides in aerial parts of Eleutherococcus species cultivated in Poland. J. AOAC. Int. 94: 1422–1426 (2011)
Zhang N, Van Crombruggen K, Holtappels G, Bachert C. A herbal composition of scutellaria baicalensis and Eleutherococcus senticosus shows potent anti-inflammatory effects in an ex vivo human mucosal tissue model. Evid. Based Complement. Alternat. Med. 2012: 673145 (2012)
Yu CY, Kim SH, Lim JD, Kim MJ, Chung IM. Intraspecific relationship analysis by DNA markers and in vitro cytotoxic and antioxidant activity in Eleutherococcus senticosus. Toxicol. In Vitro 17: 229–236 (2003)
Niu HS, Liu IM, Cheng JT, Lin CL, Hsu FL. Hypoglycemic effect of syringin from Eleutherococcus senticosus in streptozotocininduced diabetic rats. Planta Med. 74: 109–113 (2008)
Lee S, Shin DS, Oh KB, Shin KH. Antibacterial compounds from the leaves of Acanthopanax senticosus. Arch. Pharm. Res. 26: 40–42 (2003)
Jung CH, Jung H, Shin YC, Park JH, Jun CY, Kim HM, Yim HS, Shin MG, Bae HS, Kim SH, Ko SG. Eleutherococcus senticosus extract attenuates LPS-induced iNOS expression through the inhibition of Akt and JNK pathways in murine macrophage. J. Ethnopharmacol. 113: 183–187 (2007)
Hwang JS, Kim SJ, Kim HB. Development and industry of health functional food in Korea. Food Sci. Technol. Res. 15: 1–4 (2009)
Sun YL, Liu LD, Honag SK. Eleutherococcus senticosus as a crude medicine: Review of biological and pharmacological effects. J. Med. Plants Res. 5: 5946–5952 (2011)
Baczek K. Accumulation of biological active compounds in Eleuthero (Eleutherococcus senticosus/Rupr. et Maxim./Maxim.) grown in Poland. Herba Polonia 55: 7–13 (2009)
Nishibe S, Kinoshita H, Takeda H, Okano G. Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stressed rats. Chem. Pharm. Bull. 38: 1763–1765 (1990)
Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 19: R1046–R1052 (2009)
Bakan I, Laplante M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 23: 226–234 (2012)
Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4: 648–657 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, C.H., Ahn, J., Heo, S.H. et al. Eleutheroside E, an active compound from Eleutherococcus senticosus, regulates adipogenesis in 3T3-L1 cells. Food Sci Biotechnol 23, 889–893 (2014). https://doi.org/10.1007/s10068-014-0119-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-014-0119-z