Abstract
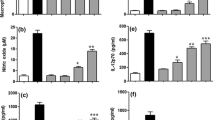
In this study, the immunemodulatory activities of arabinogalactans-type polysaccharide (APS) from Cordyceps militaris grown on germinated soybeans was evaluated in THP-1 human monocytes. The cellular size and the number of intracellular organelles of THP-1 monocytes were increased by APS. Also, APS-treated THP-1 cells significantly developed cellular adherence and macrophagic differentiation to the culture plate surface. APS noticeably enhanced phagocytic activity of THP-1 cells against IgG-FITC-latex beads. APS induced the level of TNF-α, IL-12 p40, and IL-8 as well as TLR2 and TLR4 mRNAs. APS can be developed as a promising immunmodulating agent with macrophage-activating properties.
Similar content being viewed by others
References
Puertollano MA, Puertollano E, de Cienfuegos GA, de Pablo MA. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 11: 1752–1766 (2011)
Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol. Rev. 230: 38–50 (2009)
Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol. Rev. 219: 75–87 (2007)
Mitchell GB, Albright BN, Caswell JL. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infect. Immun. 71: 1643–1649 (2003)
Werling D, Jungi TW. Toll-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunop. 91: 1–12 (2003)
Harris G, KuoLee R, Chen W. Role of Toll-like receptors in health and diseases of gastrointestinal tract. World J. Gastroentero. 12: 2149–2160 (2006)
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 327: 656–661 (2010)
Sokol RJ, Hudson G, James NT, Frost IJ, Wales J. Human macrophage development: A morphometric study. J. Anat. 151: 27–35 (1987)
Cherayil BJ, Antos D. Inducible nitric oxide synthase and Salmonella infection. Microb. Infect. 3: 771–776 (2001)
Schepetkin IA, Xie G, Kirpotina LN, Klein RA, Jutila MA, Quinn MT. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. J. Immunopharmacol. 8: 1455–1466 (2008)
Ohta Y, Lee JB, Hayashi K, Fujita A, Park DK, Hayashi T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agr. Food Chem. 55: 10194–10199 (2007)
Park HJ, Han ES, Park DK, Lee C, Lee KW. An extract of Phellinus linteus grown on germinated brown rice inhibits inflammation markers in RAW264.7 macrophages by suppressing inflammatory cytokines, chemokines, and mediators and upregulating antioxidant activity. J. Med. Food. 13: 1468–1477 (2010)
Park HJ, Han ES, Park DK. The ethyl acetate extract of PGP (Phellinus linteus grown on Panax ginseng) suppresses B16F10 melanoma cell proliferation through inducing cellular differentiation and apoptosis. J. Ethnopharmacol. 132: 115–121 (2010)
Han ES, Oh JY, Park HJ. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. J. Ethnopharmacol. 134: 703–710 (2011)
Gaurnier-Hausser A, Rothman VL, Dimitrov S, Tuszynski GP. The novel angiogenic inhibitor, angiocidin, induces differentiation of monocytes to macrophages. Cancer Res. 68: 5905–5914 (2008)
Koeffler HP, Bar-Eli M, Territo MC. Phorbol ester effect on differentiation of human myeloid leukemia cell lines blocked at different stages of maturation. Cancer Res. 41: 919–926 (1981)
Vinals M, Bermudez I, Llaverias G. Aspirin increases CD36, SR-BI, and ABCA1 expression in human THP-1 macrophages. Cardiovasc. Res. 66: 141–149 (2005)
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5: 953–964 (2005)
Delano MJ, Thayer T, Gabrilovich S. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J. Immunol. 186: 195–202 (2011)
Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 5: e8668 (2010)
Doshi N, Mitragotri S. Macrophages recognize size and shape of their targets. PLoS One 5: e10051 (2010)
Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through β-glucan-dependent pathways. J. Allergy Clin. Immun. 123: 612–618 (2009)
Pouliot P, Plante I, Raquil MA, Tessier PA, Olivier M. Myeloidrelated proteins rapidly modulate macrophage nitric oxide production during innate immune response. J. Immunol. 181: 3595–3601 (2008)
Mak NK, Fung MC, Leung KN, Hapel AJ. Monocytic differentiation of a myelomonocytic leukemic cell (WEHI 3B JCS) is induced by tumour necrosis factor-α (TNF-α). Cell Immunol. 150: 1–14 (1993
Cho HY, Choi EK, Lee SW, Kim KH, Park SJ, Lee CK. All-trans retinoic acid induces TLR-5 expression and cell differentiation and promotes flagellin-mediated cell functions in human THP-1 cells. Immunol. Lett. 136: 97–107 (2011)
Kang HJ, Ha JM, Kim HS, Lee H, Kurokawa K, Lee BL. The role of phagocytosis in IL-8 production by human monocytes in response to lipoproteins on Staphylococcus aureus. Biochem. Bioph. Res. Co. 406: 449–453 (2011)
Pascual M, Fernandez-Lizarbe S, Guerri C. Role of TLR4 in ethanol effects on innate and adaptive immune responses in peritoneal macrophages. Immunol. Cell Biol. 89: 716–727 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, D.K., Hayashi, T. & Park, HJ. Arabinogalactan-type polysaccharides (APS) from Cordyceps militaris grown on germinated soybeans (GSC) induces innate immune activity of THP-1 monocytes through promoting their macrophage differentiation and macrophage activity. Food Sci Biotechnol 21, 1501–1506 (2012). https://doi.org/10.1007/s10068-012-0199-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-012-0199-6