Abstract
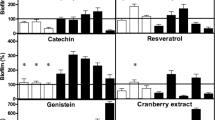
This experiment was carried out to determine the growth stimulation/inhibition effect of popular herbal extracts on intestinal microbiota and pathogenic bacteria. A paper disc agar diffusion method was employed for preliminary data. All extracts failed to promote intestinal microbiota growth around the discs. Green tea (Camellia sinensis) and Eleutherine americana did not produce inhibition zones against all intestinal microbiota, but inhibited Gram-positive pathogenic bacteria. All pure compounds, except eleutherin demonstrated antibacterial activity against all bacteria. Growth response of the substances on intestinal microbiota were further investigated by viable counts. Eleutherin from E. americana did not produce antibacterial antagonism against important groups of intestinal microbiota. In contrast, E. americana extract and eleutherin at minimal inhibitory concentration (MIC) and 4 MIC showed significant inhibition on growth of Grampositive pathogenic bacteria. The results indicated that both E. americana extract and eleutherin exerted dual beneficial effects to the host by regulating beneficial bacteria and inhibiting pathogenic bacteria.
Similar content being viewed by others
References
Macfarlane S, Macfarlane G. Gut Flora, Nutrition, Immunity, and Health. Blackwell Publishing, London, UK. pp. 127–235 (2003)
Montalto M, Onotrio FD, Gallo A, Cazzato A, Gasbarrini G. Intestinal microbiota and its functions. Digest Liver Dis. 3: 30–34 (2009)
WHO. Antimicrobial Resistance. The World Health Organization Fact Sheet. World Health Organization, Geneva, Switzerland. pp. 1–4 (2002)
Haahr V, Peterslund N, Moller J. The influence of antimicrobial prophylaxis on the microbial and clinical findings in patients after autologous bone marrow transplantation. Scand. J. Infect. Dis. 29: 623–626 (1997)
Vagionas K, Ngassapa O, Runyoro D, Graikou K, Gortzi O, Chinou I. Chemical analysis of edible aromatic plants growing in Tanzania. Food Chem. 105: 1711–1717 (2007)
WHO. Traditional Medicine. The World Health Organization Fact Sheet. World Health Organization, Geneva, Switzerland. pp. 1–3 (2003)
Bandyopadhyay D, Chatterjee T, Dasgupta A, Lourduraja J, Dastidar SG. In vitro and in vivo antimicrobial action of tea: The commonest beverage of Asia. Biol. Pharm. Bull. 28: 2125–2127 (2005)
Ifesan B, Hamtasin C, Mahabusarakam W, Voravuthikunchai S. Inhibitory effect of Eleutherine americana Merr. extract on Staphylococcus aureus isolated from food. J. Food Sci. 74: 331–336 (2009)
Ifesan B, Hamtasin C, Mahabusarakam W, Voravuthikunchai S. Assessment of antistaphylococcal activity of partially purified fractions and pure compounds from Eleutherine americana. J. Food Protect. 72: 354–359 (2009)
Li Y, Xu C, Zhang Q, Liu JY, Tan RX. In vitro anti-helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J. Ethnopharmacol. 98: 329–333 (2005)
Limsuwan S, Subhadhirasakul S, Voravuthikunchai S. Medicinal plants with significant activity against important pathogenic bacteria. Pharm Biol. 47: 683–689 (2009)
Narayana K, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y, Krishna P. Biological activity of phenylpropionic acid isolated from a terrestrial streptomycetes. Pol. J. Microbiol. 56: 191–197 (2007)
Tuncel G, Nergiz C. Antimicrobial effect of some olive phenols in a laboratory medium. Lett. Appl. Microbiol. 17: 300–302 (1993)
Voravuthikunchai S, Chusri S, Suwalak S. Quercus infectoria Oliv. Pharm. Biol. 46: 367–372 (2008)
Puupponen-Pimia R, Nohynek L, Meier C, Kahkonen M, Heinonen M, Kopia A, Oksman-Caldentey KM. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 90: 494–507 (2001)
Jeong EY, Jeon JH, Kim HW, Kim MG, Lee HS. Antimicrobial activity of leptospermone and its derivatives against human intestinal bacteria. Food Chem. 115: 1401–1404 (2009)
Park B, Kim J, Lee S, Kim KS, Takeoka GR, Ahn Y, Kim JH. Selective growth-inhibiting effects of compounds identified in Tabebuia impetiginosa inner bark on human intestinal bacteria. J. Agr. Food Chem. 53: 1152–1157 (2005)
Jeong EY, Jeon JH, Lee CH, Lee HS. Antimicrobial activity of catechol isolated from Diospyros kaki Thumb. roots and its derivatives toward intestinal bacteria. Food Chem. 115: 1006–1010 (2009)
Hervert-Hernandez D, Pintado C, Rotgu R, Goni I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 136: 119–122 (2009)
Mahabusarakam W, Hemtasin C, Chakthong S, Voravuthikunchai SP, Olawumi IB. Naphthoquinones, anthraquinones, and naphthalene derivatives from the bulbs of Eleutherine americana. Planta Med. 40: 23–27 (2009)
Krieg NR, Holt JG, Murray RGE, Brenner DJ, Bryant MP, Moulder JW. Bergey’s Manual of Systematic Bacteriology. Williams & Wilkins Baltimore, London, UK. pp. 548–667 (1984)
Forbes B, Shan D, Weissfield A. Bailey & Scott’s Diagnostic Microbiology. Mosby, St. Louis, MO, USA. pp. 127–205 (2002)
Holdeman LV, Moore WEC. Anaerobe Laboratory Manual. Virginia Polytechnic Institute, Blacksburg, VA, USA. pp. 32–69 (1977)
Woodward EJ. The effect of traditionally prepared herbal decoctions on the growth of indigenous oral microbiota. J. Sci. 3: 464–468 (1999)
Almajano MP, Carbo R, Ropez-Jimenez JA, Gordon MH. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 108: 55–63 (2008)
Lee H, Jenner A, Low C, Lee Y. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 157: 876–884 (2006)
Gury J, Barthelmebs L, Tran NP, Davies C, Cavin JF. Cloning, detection, and characterization of padR, the transcriptional repressor of the phenolic acid decarboxylase encoding padA gene of Lactobacillus plantarum. Appl. Environ. Microb. 70: 2146–2153 (2004)
Puupponen-Pimi R, Nohynek L, Hartmann-Schmidlin S, Heinonen M, Riihinen K. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 98: 991–1000 (2005)
Lim MY, Jeon JH, Jeong EY, Lee CH, Lee HS. Antimicrobial activity of 5-hydroxy-1,4-naphthoquinone isolated from Caesalpinia sappan toward intestinal bacteria. Food Chem. 100: 1254–1258 (2007)
Kamijo M, Kanazawa T, Funaki M, Nishizawa M, Yamagishi T. Effects of Rosa rugosa petals on intestinal bacteria. Biosci. Biotech. Bioch. 72: 773–777 (2008)
Sung BK, Kim MK, Lee WH, Lee DH, Lee HS. Growth responses of Cassia obtusifolia toward human intestinal bacteria. Fitoterapia 75: 505–509 (2004)
Alberto M, Gmez-Cordov C, de Nadra M. Metabolism of gallic acid and catechin by Lactobacillus hilgardii from wine. J. Agr. Food Chem. 52: 6465–6469 (2004)
Garcia-Ruiz A, Bartolom B, Martinez-Rodriguez A, Pueyo E, Martin-Alvarez P, Moreno-Arribas M. Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Control 19: 835–841 (2008)
Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics gut microbiota: Role in human health. J. Agr. Food Chem. 57: 6485–6501 (2009)
Ridwan B, Koning C, Besselink M, Timmerman H, Brouwer E, Verhoef J. Antimicrobial activity of a multispecies probiotic (Ecologic 641) against pathogens isolated from infected pancreatic necrosis. Lett. Appl. Microbiol. 46: 61–67 (2008)
Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn SV. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14: 166–171 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phoem, A.N., Voravuthikunchai, S.P. Growth stimulation/inhibition effect of medicinal plants on human intestinal microbiota. Food Sci Biotechnol 21, 739–745 (2012). https://doi.org/10.1007/s10068-012-0096-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-012-0096-z