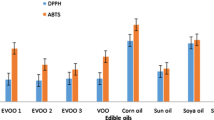
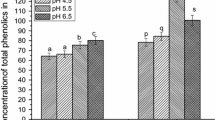
Abstract
Changes of 2,2-diphenyl-1-picrylhydrazyl (DPPH) absorbance were monitored in thermally-oxidized lard containing free radical scavengers (FRSs). Hydrogen donating abilities of α-tocopherol to DPPH were higher than those of sesamol and butylated hydroxyanisole (BHA), indicating reactivity of DPPH and FRSs in lard depends on the types of FRSs. For the first 20 min oxidation, DPPH absorbance from lard with 3.38 μmol/g α-tocopherol, sesamol, and BHA increased by 0.324, 0.240, and 0.313, respectively while those from lard without FRS decreased by 0.350. Results of conjugated dienoic acid (CDA), and p-anisidine value (p-AV) showed that antioxidative activity were high in the order of sesamol, BHA, and α-tocopherol, which implies that hydrogen donating abilites of FRSs may not be the most critical factor to determine antioxidant effectiveness. Monitoring DPPH absorbance in oxidized lard with FRS could be useful indicators for comparing antioxidative ability of FRSs.
Similar content being viewed by others
References
Boff JM, Min DB. Chemistry and reaction of singlet oxygen in foods. Comp. Rev. Food Sci. F. 1: 58–72 (2002)
Harman D. Aging. Overview. Ann. NY Acad. Sci. 928: 1–21 (2000)
Shahidi F, Naczk M. Phenolics in Food and Nutraceuticals. CRC Press, New York, NY, USA. pp. 429–431 (2004)
Decker EA. Antioxidant mechanisms. pp. 397–401. In: Food Lipids. Akoh CC, Min DB (eds). Marcel Dekker, New York, NY, USA (2002)
Lee JM, Chung H, Chang PS, Lee JH. Development of a method predicting the oxidative stability of edible oils using 2,2-diphenyl-1-picrylhydrazyl (DPPH). Food Chem. 103: 662–669 (2007)
Shahidi F, Wanasundara UN. Methods of measuring oxidative rancidity in fats and oils. pp. 377–396. In: Food Lipids. Akoh CC, Min DB (eds). Marcel Dekker, New York, NY, USA (1998)
Alamed J, Julian M, Decker EA. Influence of heat processing and calcium ions on the ability of EDTA to inhibit lipid oxidation in oil-in-water emulsions containing omega-3 fatty acids. Food Chem. 95: 585–590 (2006)
Estevez M, Cava R. Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: Contradictory effects in different types of frankfurters. Meat Sci. 72: 348–355 (2006)
Lee JM, Kim DH, Chang PS, Lee JH. Headspace-solid phase microextraction (HS-SPME) analysis of oxidized volatiles from free fatty acids (FFA) and application for measuring hydrogen donating antioxidant activity. Food Chem. 104: 414–420 (2007)
AOCS. Official Methods and Recommended Practices of the AOCS. Method Ti 1a-64. American Oil Chemists Society, Champaign, IL, USA (1980)
AOCS. Official Methods and Recommended Practices of the AOCS. Method Cd 18-90. American Oil Chemists Society, Champaign, IL, USA (1990)
Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 103: 1494–1501 (2007)
Liu DH, Shi J, Ibarra AC, Kakuda Y, Xue SJ. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT -Food Sci. Technol. 41: 1344–1349 (2008)
Wettasinghe M, Shahidi F. Scavenging of reactive-oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chem. 70: 7–26 (2000)
Gordon MH, Kourimska L. The effects of antioxidants on changes in oils during heating and deep frying. J. Sci. Food Agr. 68: 347–353 (1995)
Sharma GK, Semwal AD, Narashimha MC, Arya SS. Stability of antioxygenic salts for stabilization of fried snacks. Food Chem. 60: 19–24 (1997)
Khan MA, Shahidi F. Effects of natural and synthetic antioxidants on the oxidative stability of borage and evening primrose triacylglycerols. Food Chem. 75: 431–437 (2001)
Lee SW, Jeung MK, Park MH, Lee SY, Lee JH. Effects of roasting conditions of sesame seeds on the oxidative stability of pressed oil during thermal oxidation. Food Chem. 118: 681–685 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeo, J., Jeong, M.K. & Lee, J. Application of DPPH absorbance method to monitor the degree of oxidation in thermally-oxidized oil model system with antioxidants. Food Sci Biotechnol 19, 253–256 (2010). https://doi.org/10.1007/s10068-010-0036-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-010-0036-8