Abstract
Objective
To evaluate the safety of secukinumab (SEC) in the treatment of patients with axial spondyloarthritis (axSpA) and concurrent hepatitis B virus (HBV) infection or latent tuberculosis infection (LTBI).
Methods
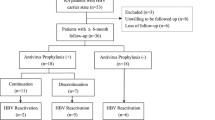
This is a retrospective cohort study. Adult axSpA patients with HBV infection or LTBI receiving SEC treatment for at least 3 months from March 2020 to July 2022 in Guangdong Provincial People’s Hospital were included. Patients were screened for HBV infection and LTBI before SEC treatment. During follow-up, reactivation of HBV infection and LTBI was monitored. Relevant data were collected and analyzed.
Results
A total of 43 axSpA patients with HBV infection or LTBI were included, of whom 37 were with HBV infection, 6 were with LTBI. Six out of thirty-seven (16.2%) patients with axSpA and concurrent HBV infection exhibited HBV reactivation after 9.0 ± 5.7 months of SEC treatment. Among them, 3 patients had chronic HBV infection and received anti-HBV prophylaxis, 2 patients had chronic HBV infection but did not receive anti-HBV prophylaxis, and 1 patient had occult HBV infection and did not receive antiviral prophylaxis. None of the 6 axSpA patients with LTBI developed reactivation of LTBI, whether received anti-TB prophylaxis or not.
Conclusions
HBV reactivation can occur in axSpA patients with different types of HBV infection undergoing SEC treatment, whether receive antiviral prophylaxis or not. Close monitoring of HBV reactivation in axSpA patients with HBV infection undergoing SEC treatment is mandatory. Anti-HBV prophylaxis may be beneficial. In contrast, SEC may be safe in axSpA patients with LTBI, even in patients not receiving anti-TB prophylaxis.
Key Points •Currently, most evidence about the safety of SEC in patients with HBV infection and LTBI were from patients with psoriasis. Our study adds data about the safety of SEC in Chinese axSpA patients with concurrent HBV infection or LTBI in real-world clinical setting. •Our study showed that HBV reactivation can occur in axSpA patients with different types of HBV infection undergoing SEC treatment, whether receive antiviral prophylaxis or not. •Close monitoring of serum HBV markers, HBV DNA load, and liver function is mandatory in axSpA patients with chronic, occult, and resolved HBV infection undergoing SEC treatment. Anti-HBV prophylaxis may be beneficial in all HBsAg-positive patients and HBsAg-negative, HBcAb-positive patients at high risk of HBV reactivation who are receiving SEC therapy. •None of the axSpA patients with LTBI, whether received anti-TB prophylaxis or not, developed reactivation of LTBI in our study. SEC may be safe in axSpA patients with LTBI, even in patients not receiving anti-TB prophylaxis. |


Similar content being viewed by others
Data availability
The datasets generated and analyzed in our study are available from the corresponding author on reasonable request.
References
Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet 390:73–84. https://doi.org/10.1016/s0140-6736(16)31591-4
Robinson PC, van der Linden S, Khan MA, Taylor WJ (2021) Axial spondyloarthritis: concept, construct, classification and implications for therapy. Nat Rev Rheumatol 17:109–118. https://doi.org/10.1038/s41584-020-00552-4
Ward MM, Deodhar A et al (2019) 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol 71:1599–1613. https://doi.org/10.1002/art.41042
Baeten D, Sieper J et al (2015) Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 373:2534–2548. https://doi.org/10.1056/NEJMoa1505066
van der Heijde D, Ramiro S et al (2017) 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 76:978–991. https://doi.org/10.1136/annrheumdis-2016-210770
Cantini F, Nannini C et al (2015) Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev 14:503–509. https://doi.org/10.1016/j.autrev.2015.01.011
Cannizzaro MV, Franceschini C, Esposito M, Bianchi L, Giunta A (2017) Hepatitis B reactivation in psoriasis patients treated with anti-TNF agents: prevention and management. Psoriasis 7:35–40. https://doi.org/10.2147/ptt.S108209
Pauly MP, Tucker LY et al (2018) Incidence of hepatitis B virus reactivation and hepatotoxicity in patients receiving long-term treatment with tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 16:1964-1973.e1. https://doi.org/10.1016/j.cgh.2018.04.033
Elewski BE, Baddley JW et al (2021) Association of secukinumab treatment with tuberculosis reactivation in patients with psoriasis, psoriatic arthritis, or ankylosing spondylitis. JAMA Dermatol 157:43–51. https://doi.org/10.1001/jamadermatol.2020.3257
Fowler E, Ghamrawi RI, Ghiam N, Liao W, Wu JJ (2020) Risk of tuberculosis reactivation during interleukin-17 inhibitor therapy for psoriasis: a systematic review. J Eur Acad Dermatol Venereol 34:1449–1456. https://doi.org/10.1111/jdv.16254
Chiu HY, Hui RC et al (2018) Safety profile of secukinumab in treatment of patients with psoriasis and concurrent hepatitis B or C: a multicentric prospective cohort study. Acta Derm Venereol 98:829–834. https://doi.org/10.2340/00015555-2989
Zheng B, Li T et al (2012) Prevalence of hepatitis B surface antigen in patients with ankylosing spondylitis and its association with HLA-B27: a retrospective study from south China. Rheumatol Int 32:2011–2016. https://doi.org/10.1007/s00296-011-1934-7
Liu YJ, Xu J et al (2019) The prevalence of latent tuberculosis infection in patients with inflammatory arthritis and the diagnostic efficacy of different screening methods. Zhonghua Yi Xue Za Zhi 99:20–24. https://doi.org/10.3760/cma.j.issn.0376-2491.2019.01.005
Rudwaleit M, van der Heijde D et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68:777–783. https://doi.org/10.1136/ard.2009.108233
Piaserico S, Messina F, Russo FP (2019) Managing psoriasis in patients with HBV or HCV infection: practical considerations. Am J Clin Dermatol 20:829–845. https://doi.org/10.1007/s40257-019-00457-3
Chee CBE, Reves R, Zhang Y, Belknap R (2018) Latent tuberculosis infection: opportunities and challenges. Respirology 23:893–900. https://doi.org/10.1111/resp.13346
Cantini F, Nannini C et al (2017) Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediators Inflamm 2017:8909834. https://doi.org/10.1155/2017/8909834
Haas MK, Belknap RW (2019) Diagnostic tests for latent tuberculosis infection. Clin Chest Med 40:829–837. https://doi.org/10.1016/j.ccm.2019.07.007
Lee E, Holzman RS (2002) Evolution and current use of the tuberculin test. Clin Infect Dis 34:365–370. https://doi.org/10.1086/338149
Pai M, Riley LW, Colford JM Jr (2004) Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 4:761–776. https://doi.org/10.1016/s1473-3099(04)01206-x
Shi Y, Zheng M (2020) Hepatitis B virus persistence and reactivation. Bmj 370:m2200. https://doi.org/10.1136/bmj.m2200
Gong W, Wu X (2021) Differential diagnosis of latent tuberculosis infection and active tuberculosis: a key to a successful tuberculosis control strategy. Front Microbiol 12:745592. https://doi.org/10.3389/fmicb.2021.745592
Megna M, Patruno C et al (2022) Hepatitis virus reactivation in patients with psoriasis treated with secukinumab in a real-world setting of hepatitis B or hepatitis C infection. Clin Drug Investig 42:525–531. https://doi.org/10.1007/s40261-022-01163-5
Qin H, Liu N et al (2022) Safety and efficacy of secukinumab in psoriasis patients infected with hepatitis B virus: a retrospective study. Eur J Dermatol 32:394–400. https://doi.org/10.1684/ejd.2022.4263
EASL (2017) Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 67:370–398. https://doi.org/10.1016/j.jhep.2017.03.021
Megna M, Patruno C et al (2022) Lack of reactivation of tuberculosis in patients with psoriasis treated with secukinumab in a real-world setting of latent tuberculosis infection. J Dermatolog Treat 33:2629–2633. https://doi.org/10.1080/09546634.2022.2062280
Shu D, Zhang Z, Zhou EY, Ma X, Zhao Y (2020) Is chemoprophylaxis necessary for all latent tuberculosis infection patients receiving IL-17 inhibitors? A cohort study. Dermatol Ther 33:e14512. https://doi.org/10.1111/dth.14512
Segueni N, Tritto E et al (2016) Controlled Mycobacterium tuberculosis infection in mice under treatment with anti-IL-17A or IL-17F antibodies, in contrast to TNFα neutralization. Sci Rep 6:36923. https://doi.org/10.1038/srep36923
Huang Z, van Velkinburgh JC, Ni B, Wu Y (2012) Pivotal roles of the interleukin-23/T helper 17 cell axis in hepatitis B. Liver Int 32:894–901. https://doi.org/10.1111/j.1478-3231.2012.02764.x
Author information
Authors and Affiliations
Contributions
Yang Cui had the original idea of the study. All authors contributed to the study design. Suling Liu, Ziye He, Wenjing Wu, and Hua Jin collected the data. Suling Liu analyzed and interpreted the data, searched the literature, and drafted the manuscript. All authors revised the manuscript, gave the final approval of the submitted version of the manuscript, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
This study received approval from the institutional review board of Guangdong Provincial People’s Hospital with the ethics number of KY-Q-2021–164 and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was waived because the data were collected retrospectively and only anonymized data were used in the analysis.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., He, Z., Wu, W. et al. Safety of secukinumab in the treatment of patients with axial spondyloarthritis and concurrent hepatitis B virus infection or latent tuberculosis infection. Clin Rheumatol 42, 2369–2376 (2023). https://doi.org/10.1007/s10067-023-06630-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06630-8