Abstract
Background
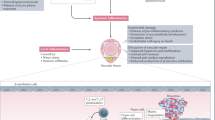
Rheumatoid arthritis (RA) increases the risk of cardiovascular disease (CVD), with inflammation playing a key role. Biologic and targeted synthetic drugs used to treat RA can induce systemic immunomodulation and may have pleiotropic effects on vascular function, making it crucial to investigate their impact on CVD risk in RA patients.
Methods
A systematic review of the literature was conducted to investigate the impact of biologic and targeted synthetic treatments approved for RA on various cardiovascular markers, including endothelial function, arterial stiffness, and subclinical atherosclerosis. Our analysis included a search of the MedLine (via PubMed) and Web of Science databases using a pre-determined search strategy. We conducted a narrative synthesis of the included studies due to heterogeneity in study design and outcome measures.
Results
From an initial pool of 647 records, we excluded 327 studies based on their titles and abstracts, and we selected 182 studies for final examination. Ultimately, 58 articles met our inclusion criteria and were included in our systematic review. Our analysis of these studies revealed a positive effect of biologic and targeted synthetic therapies on vascular dysfunction associated with RA. However, the impact of these treatments on subclinical atherosclerosis was inconsistent.
Conclusion
Overall, our systematic review provides important insights into the potential cardiovascular benefits of biologic and targeted synthetic treatments for RA by a still unknown mechanism. These findings can inform clinical practice and contribute to our understanding of their possible effects on early vascular pathology.
Key Points • Great heterogeneity of methods are used to evaluate the endothelial function and arterial stiffness in patients with RA on biologic and targeted synthetic antirheumatic drugs. • Most studies have shown a considerable improvement in endothelial function and arterial stiffness with TNFi, despite some studies reporting only transient or no improvement. • Anakinra and tocilizumab may have a beneficial effect on vascular function and endothelial injury, as indicated by increased FMD, coronary flow reserve, and reduced levels of biomarkers of endothelial function, while the overall impact of JAKi and rituximab remains inconclusive based on the reviewed studies. • To fully comprehend the distinctions between biologic therapies, more long-term, well-designed clinical trials are necessary using a homogeneous methodology. |

Similar content being viewed by others
References
Roth GA, Johnson C, Abajobir A et al (2017) Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 70:1–25. https://doi.org/10.1016/j.jacc.2017.04.052
Ross R (1999) Atherosclerosis — An Inflammatory Disease. N EnglJ Med 340:115–126. https://doi.org/10.1056/NEJM199901143400207
Ridker PM (2019) Anticytokine Agents. Circ Res 124:437–450. https://doi.org/10.1161/CIRCRESAHA.118.313129
Tocci G, Goletti D, Marino V et al (2016) Cardiovascular outcomes and tumour necrosis factor antagonists in chronic inflammatory rheumatic disease: a focus on rheumatoid arthritis. Expert Opin Drug Saf 15:55–61. https://doi.org/10.1080/14740338.2016.1218469
Tanaka T, Kishimoto T (2014) The Biology and Medical Implications of Interleukin-6. Cancer Immunol Res 2:288–294. https://doi.org/10.1158/2326-6066.CIR-14-0022
Meyer PW, Anderson R, Ker JA, Ally MT (2018) Rheumatoid arthritis and risk of cardiovascular disease. Cardiovasc J Afr 29:317–321. https://doi.org/10.5830/CVJA-2018-018
Almutairi K, Nossent J, Preen D et al (2021) The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 41:863–877. https://doi.org/10.1007/s00296-020-04731-0
Løgstrup BB, Ellingsen T, Pedersen AB et al (2021) Cardiovascular risk and mortality in rheumatoid arthritis compared with diabetes mellitus and the general population. Rheumatology 60:1400–1409. https://doi.org/10.1093/rheumatology/keaa374
Agca R, Heslinga SC, Rollefstad S et al (2017) EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 76:17–28. https://doi.org/10.1136/annrheumdis-2016-209775
Visseren FLJ, Mach F, Smulders YM et al (2021) 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 42:3227–3337. https://doi.org/10.1093/eurheartj/ehab484
Arida A, Protogerou A, Kitas G, Sfikakis P (2018) Systemic Inflammatory Response and Atherosclerosis: The Paradigm of Chronic Inflammatory Rheumatic Diseases. Int J Mol Sci 19:1890. https://doi.org/10.3390/ijms19071890
Ridker PM, Rane M (2021) Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ Res 128:1728–1746. https://doi.org/10.1161/CIRCRESAHA.121.319077
Murdaca G, Colombo BM, Cagnati P et al (2012) Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 224:309–317. https://doi.org/10.1016/j.atherosclerosis.2012.05.013
Poredos P, Poredos AV, Gregoric I (2021) Endothelial Dysfunction and Its Clinical Implications. Angiology 72:604–615. https://doi.org/10.1177/0003319720987752
Smolen JS, Landewé RBM, Bijlsma JWJ et al (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79:685–699. https://doi.org/10.1136/annrheumdis-2019-216655
Xie F, Yun H, Levitan EB et al (2019) Tocilizumab and the Risk of Cardiovascular Disease: Direct Comparison Among Biologic Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis Patients. Arthritis Care Res (Hoboken) 71:1004–1018. https://doi.org/10.1002/acr.23737
Singh S, Fumery M, Singh AG et al (2020) Comparative Risk of Cardiovascular Events With Biologic and Synthetic Disease-Modifying Antirheumatic Drugs in Patients With Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken) 72:561–576. https://doi.org/10.1002/acr.23875
Naranjo A, Sokka T, Descalzo MA et al (2008) Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 10:R30. https://doi.org/10.1186/ar2383
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409–1417. https://doi.org/10.1007/s00296-011-1999-3
Hürlimann D, Forster A, Noll G et al (2002) Anti–Tumor Necrosis Factor-α Treatment Improves Endothelial Function in Patients With Rheumatoid Arthritis. Circulation 106:2184–2187. https://doi.org/10.1161/01.CIR.0000037521.71373.44
Hänsel S, Lässig G, Pistrosch F, Passauer J (2003) Endothelial dysfunction in young patients with long-term rheumatoid arthritis and low disease activity. Atherosclerosis 170:177–180. https://doi.org/10.1016/S0021-9150(03)00281-8
Gonzalez-Juanatey C, Testa A, Garcia-Castelo A et al (2004) Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long-term treatment with anti-tumor necrosis factor α antibody. Arthritis Care Res (Hoboken) 51:447–450. https://doi.org/10.1002/art.20407
Irace C, Mancuso G, Fiaschi E et al (2004) Effect of anti TNFalpha therapy on arterial diameter and wall shear stress and HDL cholesterol. Atherosclerosis 177:113–118. https://doi.org/10.1016/j.atherosclerosis.2004.04.031
van Doornum S, McColl G, Wicks IP (2005) Tumour necrosis factor antagonists improve disease activity but not arterial stiffness in rheumatoid arthritis. Rheumatology 44:1428–1432. https://doi.org/10.1093/rheumatology/kei033
Gonzalez-Gay MA, Garcia-Unzueta MT, de Matias JM et al (2006) Influence of anti-TNF-alpha infliximab therapy on adhesion molecules associated with atherogenesis in patients with rheumatoid arthritis. Clin Exp Rheumatol 24:373–379
Bilsborough W, Keen H, Taylor A et al (2006) Anti-tumour necrosis factor-alpha therapy over conventional therapy improves endothelial function in adults with rheumatoid arthritis. Rheumatol Int 26:1125–1131. https://doi.org/10.1007/s00296-006-0147-y
Gonzalez-Juanatey C, Llorca J, Sanchez-Andrade A et al (2006) Short-term adalimumab therapy improves endo-thelial function in patients with rheumatoid arthritis refractory to infliximab. Clin Exp Rheumatol 24:309–312
Mäki-Petäjä KM, Hall FC, Booth AD et al (2006) Rheumatoid Arthritis Is Associated With Increased Aortic Pulse-Wave Velocity, Which Is Reduced by Anti–Tumor Necrosis Factor-α Therapy. Circulation 114:1185–1192. https://doi.org/10.1161/CIRCULATIONAHA.105.601641
Komai N, Morita Y, Sakuta T et al (2007) Anti-tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol 17:385–390. https://doi.org/10.1007/s10165-007-0605-8
Cypiene A, Laucevicius A, Venalis A et al (2007) Non-invasive assessment of arterial stiffness indices by applanation tonometry and pulse wave analysis in patients with rheumatoid arthritis treated with TNF-alpha blocker remicade (infliximab). Proc West Pharmacol Soc 50:119–122
del Porto F, Lagana B, Lai S et al (2007) Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology 46:1111–1115. https://doi.org/10.1093/rheumatology/kem089
Bosello S, Santoliquido A, Zoli A et al (2008) TNF-alpha blockade induces a reversible but transient effect on endothelial dysfunction in patients with long-standing severe rheumatoid arthritis. Clin Rheumatol 27:833–839. https://doi.org/10.1007/s10067-007-0803-y
Wong M, Oakley SP, Young L et al (2009) Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis 68:1277–1284. https://doi.org/10.1136/ard.2007.086157
Sidiropoulos PI, Siakka P, Pagonidis K et al (2009) Sustained improvement of vascular endothelial function during anti-TNFα treatment in rheumatoid arthritis patients. Scand J Rheumatol 38:6–10. https://doi.org/10.1080/03009740802363768
Capria A, de Nardo D, Baffetti FR et al (2010) Long-Term Anti-TNF-α Treatments Reverse the Endothelial Dysfunction in Rheumatoid Arthritis: The Biological Coherence between Synovial and Endothelial Inflammation. Int J Immunopathol Pharmacol 23:255–262. https://doi.org/10.1177/039463201002300123
Galarraga B, Khan F, Kumar P et al (2009) Etanercept improves inflammation-associated arterial stiffness in rheumatoid arthritis. Rheumatology 48:1418–1423. https://doi.org/10.1093/rheumatology/kep251
Klimiuk P, Sierakowski S, Domyslawska I, Chwiecko J (2009) Effect of etanercept on serum levels of soluble cell adhesion molecules (sICAM-1, sVCAM-1, and sE-selectin) and vascular endothelial growth factor in patients with rheumatoid arthritis. Scand J Rheumatol 38:439–444. https://doi.org/10.3109/03009740903079321
Turiel M, Tomasoni L, Sitia S et al (2010) RESEARCH: Effects of Long-Term Disease-Modifying Antirheumatic Drugs on Endothelial Function in Patients with Early Rheumatoid Arthritis. Cardiovasc Ther 28:e53–e64. https://doi.org/10.1111/j.1755-5922.2009.00119.x
Tikiz H, Arslan O, Pirildar T et al (2010) The effect of anti-tumor necrosis factor (TNF)-alpha therapy with etanercept on endothelial functions in patients with rheumatoid arthritis. Anadolu Kardiyoloji Dergisi/The Anatolian Journal of Cardiology 10:98–103. https://doi.org/10.5152/akd.2010.031
Galarraga B, JJF B, Pullar T et al (2010) Clinical Improvement in Rheumatoid Arthritis Is Associated with Healthier Microvascular Function in Patients Who Respond to Antirheumatic Therapy. J Rheumatol 37:521–528. https://doi.org/10.3899/jrheum.090417
Ajeganova S, Fiskesund R, de Faire U et al (2011) Effect of biological therapy on levels of atheroprotective antibodies against phosphorylcholine and apolipoproteins in rheumatoid arthritis - a one year study. Clin Exp Rheumatol 29:942–950
Kerekes G, Soltész P, Szucs G et al (2011) Effects of adalimumab treatment on vascular disease associated with early rheumatoid arthritis. Isr Med Assoc J 13:147–152
Kume K, Amano K, Yamada S et al (2011) Tocilizumab Monotherapy Reduces Arterial Stiffness as Effectively as Etanercept or Adalimumab Monotherapy in Rheumatoid Arthritis: An Open-label Randomized Controlled Trial. J Rheumatol 38:2169–2171. https://doi.org/10.3899/jrheum.110340
Tam L-S, Shang Q, Li EK et al (2012) Infliximab is Associated with Improvement in Arterial Stiffness in Patients with Early Rheumatoid Arthritis — A Randomized Trial. J Rheumatol 39:2267–2275. https://doi.org/10.3899/jrheum.120541
Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA et al (2012) Anti-TNF-Alpha-Adalimumab Therapy Is Associated with Persistent Improvement of Endothelial Function without Progression of Carotid Intima-Media Wall Thickness in Patients with Rheumatoid Arthritis Refractory to Conventional Therapy. Mediators Inflamm 2012:1–8. https://doi.org/10.1155/2012/674265
Hjeltnes G, Hollan I, Førre O et al (2013) Serum levels of lipoprotein(a) and E-selectin are reduced in rheumatoid arthritis patients treated with methotrexate or methotrexate in combination with TNF-α-inhibitor. Clin Exp Rheumatol 31:415–421
Daïen CI, Fesler P, du Cailar G et al (2013) Etanercept normalises left ventricular mass in patients with rheumatoid arthritis. Ann Rheum Dis 72:881–887. https://doi.org/10.1136/annrheumdis-2012-201489
Mäki-Petäjä KM, Elkhawad M, Cheriyan J et al (2012) Anti-Tumor Necrosis Factor-α Therapy Reduces Aortic Inflammation and Stiffness in Patients With Rheumatoid Arthritis. Circulation 126:2473–2480. https://doi.org/10.1161/CIRCULATIONAHA.112.120410
Spinelli FR, Metere A, Barbati C et al (2013) Effect of Therapeutic Inhibition of TNF on Circulating Endothelial Progenitor Cells in Patients with Rheumatoid Arthritis. Mediators Inflamm 2013:1–8. https://doi.org/10.1155/2013/537539
Spinelli FR, di Franco M, Metere A et al (2014) Decrease of Asymmetric Dimethyl Arginine After Anti-TNF Therapy in Patients with Rheumatoid Arthritis. Drug Dev Res 75:S67–S69. https://doi.org/10.1002/ddr.21200
Vassilopoulos D, Gravos A, Vlachopoulos C et al (2015) Adalimumab decreases aortic stiffness independently of its effect in disease activity in patients with rheumatoid arthritis. Clin Rheumatol 34:359–364. https://doi.org/10.1007/s10067-014-2718-8
Rongen GA, van Ingen I, Kok M et al (2018) Vasodilator function worsens after cessation of tumour necrosis factor inhibitor therapy in patients with rheumatoid arthritis only if a flare occurs. Clin Rheumatol 37:909–916. https://doi.org/10.1007/s10067-017-3961-6
Vlachopoulos C, Gravos A, Georgiopoulos G et al (2018) The effect of TNF-a antagonists on aortic stiffness and wave reflections: a meta-analysis. Clin Rheumatol 37:515–526. https://doi.org/10.1007/s10067-017-3657-y
Dávida L, Pongrácz V, Mohamed EA et al (2020) A prospective, longitudinal monocentric study on laser Doppler imaging of microcirculation: comparison with macrovascular pathophysiology and effect of adalimumab treatment in early rheumatoid arthritis. Rheumatol Int 40:415–424. https://doi.org/10.1007/s00296-019-04503-5
Plein S, Erhayiem B, Fent G et al (2020) Cardiovascular effects of biological versus conventional synthetic disease-modifying antirheumatic drug therapy in treatment-naïve, early rheumatoid arthritis. Ann Rheum Dis 79:1414–1422. https://doi.org/10.1136/annrheumdis-2020-217653
Blanken AB, Agca R, van Sijl AM et al (2021) Arterial wall inflammation in rheumatoid arthritis is reduced by anti-inflammatory treatment. Semin Arthritis Rheum 51:457–463. https://doi.org/10.1016/j.semarthrit.2021.03.008
Anghel D, Sîrbu C, Hoinoiu E-M et al (2021) Influence of anti-TNF therapy and homocysteine level on carotid intima-media thickness in rheumatoid arthritis patients. Exp Ther Med 23:59. https://doi.org/10.3892/etm.2021.10981
Blanken AB, Raadsen R, Agca R et al (2022) Effect of anti-inflammatory therapy on vascular biomarkers for subclinical cardiovascular disease in rheumatoid arthritis patients. Rheumatol Int 43(2):315–322. https://doi.org/10.1007/s00296-022-05226-w
Szeremeta A, Jura-Półtorak A, Zoń-Giebel A et al (2022) TNF-α Inhibitors in Combination with MTX Reduce Circulating Levels of Heparan Sulfate/Heparin and Endothelial Dysfunction Biomarkers (sVCAM-1, MCP-1, MMP-9 and ADMA) in Women with Rheumatoid Arthritis. J Clin Med 11:4213. https://doi.org/10.3390/jcm11144213
Mathieu S, Pereira B, Dubost J-J et al (2012) No significant change in arterial stiffness in RA after 6 months and 1 year of rituximab treatment. Rheumatology 51:1107–1111. https://doi.org/10.1093/rheumatology/kes006
Provan SA, Berg IJ, Hammer HB et al (2015) The Impact of Newer Biological Disease Modifying Anti-Rheumatic Drugs on Cardiovascular Risk Factors: A 12-Month Longitudinal Study in Rheumatoid Arthritis Patients Treated with Rituximab. Abatacept and Tociliziumab. PLoS One 10:e0130709. https://doi.org/10.1371/journal.pone.0130709
Benucci M, Saviola M et al (2013) Factors correlated with improvement of endothelial dysfunction during rituximab therapy in patients with rheumatoid arthritis. Biologics 69. https://doi.org/10.2147/BTT.S39182
Ikonomidis I, Lekakis JP, Nikolaou M et al (2008) Inhibition of Interleukin-1 by Anakinra Improves Vascular and Left Ventricular Function in Patients With Rheumatoid Arthritis. Circulation 117:2662–2669. https://doi.org/10.1161/CIRCULATIONAHA.107.731877
Ikonomidis I, Tzortzis S, Lekakis J et al (2009) Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart 95:1502–1507. https://doi.org/10.1136/hrt.2009.168971
Ikonomidis I, Tzortzis S, Andreadou I et al (2014) Increased Benefit of Interleukin-1 Inhibition on Vascular Function, Myocardial Deformation, and Twisting in Patients With Coronary Artery Disease and Coexisting Rheumatoid Arthritis. Circ Cardiovasc Imaging 7:619–628. https://doi.org/10.1161/CIRCIMAGING.113.001193
Gonzalez-Juanatey C, Llorca J, Vazquez-Rodriguez TR et al (2008) Short-term improvement of endothelial function in rituximab-treated rheumatoid arthritis patients refractory to tumor necrosis factor α blocker therapy. Arthritis Rheum 59:1821–1824. https://doi.org/10.1002/art.24308
Kerekes G, Soltész P, Dér H et al (2009) Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol 28:705–710. https://doi.org/10.1007/s10067-009-1095-1
Hsue PY, Scherzer R, Grunfeld C et al (2014) Depletion of B-Cells With Rituximab Improves Endothelial Function and Reduces Inflammation Among Individuals With Rheumatoid Arthritis. J Am Heart Assoc 3. https://doi.org/10.1161/JAHA.114.001267
Protogerou AD, Zampeli E, Fragiadaki K et al (2011) A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis 219:734–736. https://doi.org/10.1016/j.atherosclerosis.2011.09.015
McInnes IB, Thompson L, Giles JT et al (2015) Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 74:694–702. https://doi.org/10.1136/annrheumdis-2013-204345
Bacchiega BC, Bacchiega AB, Usnayo MJG et al (2017) Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J Am Heart Assoc 6. https://doi.org/10.1161/JAHA.116.005038
Ikonomidis I, Pavlidis G, Katsimbri P et al (2019) Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin Res Cardiol 108:1093–1101. https://doi.org/10.1007/s00392-019-01443-9
Ikonomidis I, Pavlidis G, Katsimbri P et al (2020) Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem Toxicol 145:111694. https://doi.org/10.1016/j.fct.2020.111694
Kume K, Amano K, Yamada S et al (2017) Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: a cohort study. Rheumatol Int 37:2079–2085. https://doi.org/10.1007/s00296-017-3844-9
Soós B, Hamar A, Pusztai A et al (2022) Effects of tofacitinib therapy on arginine and methionine metabolites in association with vascular pathophysiology in rheumatoid arthritis: A metabolomic approach. Front Med (Lausanne) 9. https://doi.org/10.3389/fmed.2022.1011734
Czókolyová M, Hamar A, Pusztai A et al (2022) Effects of One-Year Tofacitinib Therapy on Lipids and Adipokines in Association with Vascular Pathophysiology in Rheumatoid Arthritis. Biomolecules 12:1483. https://doi.org/10.3390/biom12101483
Yuri Gasparyan A, Stavropoulos-Kalinoglou AP, Mikhailidis D et al (2010) The Rationale for Comparative Studies of Accelerated Atherosclerosis in Rheumatic Diseases. Curr Vasc Pharmacol 8:437–449. https://doi.org/10.2174/157016110791330852
Bots ML, Dijk JM, Oren A, Grobbee DE (2002) Carotid intima–media thickness, arterial stiffness and risk of cardiovascular disease. J Hypertens 20:2317–2325. https://doi.org/10.1097/00004872-200212000-00002
Bordy R, Totoson P, Prati C et al (2018) Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol 14:404–420. https://doi.org/10.1038/s41584-018-0022-8
Prati C, Demougeot C, Guillot X et al (2014) Endothelial dysfunction in joint disease. Joint Bone Spine 81:386–391. https://doi.org/10.1016/j.jbspin.2014.01.014
Wilkinson IB, Webb DJ (2001) Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 52:631–646. https://doi.org/10.1046/j.0306-5251.2001.01495.x
Puissant C, Abraham P, Durand S et al (2013) Reproducibility of Non-Invasive Assessment of Skin Endothelial Function Using Laser Doppler Flowmetry and Laser Speckle Contrast Imaging. PLoS One 8:e61320. https://doi.org/10.1371/journal.pone.0061320
Cerny V, Astapenko D, Burkovskiy I et al (2017) Glycocalyx in vivo measurement. Clin Hemorheol Microcirc 67:499–503. https://doi.org/10.3233/CH-179235
van Bortel LM, Laurent S, Boutouyrie P et al (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30:445–448. https://doi.org/10.1097/HJH.0b013e32834fa8b0
Stone K, Fryer S, Faulkner J et al (2021) Acute Changes in Carotid-Femoral Pulse-Wave Velocity Are Tracked by Heart-Femoral Pulse-Wave Velocity. Front Cardiovasc Med 7. https://doi.org/10.3389/fcvm.2020.592834
Sang T, Lv N, Dang A et al (2021) Brachial-ankle pulse wave velocity and prognosis in patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Hypertension Research 44:1175–1185. https://doi.org/10.1038/s41440-021-00678-2
Saiki A, Ohira M, Yamaguchi T et al (2020) New Horizons of Arterial Stiffness Developed Using Cardio-Ankle Vascular Index (CAVI). J Atheroscler Thromb 27:732–748. https://doi.org/10.5551/jat.RV17043
Messroghli DR, Moon JC, Ferreira VM et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19:75. https://doi.org/10.1186/s12968-017-0389-8
Swoboda PP, Erhayiem B, Kan R et al (2018) Cardiovascular magnetic resonance measures of aortic stiffness in asymptomatic patients with type 2 diabetes: association with glycaemic control and clinical outcomes. Cardiovasc Diabetol 17:35. https://doi.org/10.1186/s12933-018-0681-4
Maroules CD, Khera A, Ayers C et al (2014) Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson 16:33. https://doi.org/10.1186/1532-429X-16-33
Bots ML, Hofman A, Grobbee DE (1997) Increased Common Carotid Intima-Media Thickness. Stroke 28:2442–2447. https://doi.org/10.1161/01.STR.28.12.2442
Corrales A, González-Juanatey C, Peiró ME et al (2014) Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis 73:722–727. https://doi.org/10.1136/annrheumdis-2012-203101
Figueroa AL, Abdelbaky A, Truong QA et al (2013) Measurement of Arterial Activity on Routine FDG PET/CT Images Improves Prediction of Risk of Future CV Events. JACC Cardiovasc Imaging 6:1250–1259. https://doi.org/10.1016/j.jcmg.2013.08.006
Rose S, Sheth NH, Baker JF et al (2013) A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis 3:273–278
Geraldino-Pardilla L, Zartoshti A, Bag Ozbek A et al (2018) Arterial Inflammation Detected With 18 F-Fluorodeoxyglucose–Positron Emission Tomography in Rheumatoid Arthritis. Arthritis & Rheumatol 70:30–39. https://doi.org/10.1002/art.40345
Dowsett L, Higgins E, Alanazi S et al (2020) ADMA: A Key Player in the Relationship between Vascular Dysfunction and Inflammation in Atherosclerosis. J Clin Med 9:3026. https://doi.org/10.3390/jcm9093026
Blann A (2003) The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J 24:2166–2179. https://doi.org/10.1016/j.ehj.2003.08.021
Littler AJ, Buckley CD, Wordsworth P et al (1997) A distinct profile of six soluble adhesion molecules (ICAM-1, ICAM-3, VCAM-1, E-selectin, L-selectin and P-selectin) in rheumatoid arthritis. Rheumatology 36:164–169. https://doi.org/10.1093/rheumatology/36.2.164
Carter RA, Wicks IP (2001) Vascular cell adhesion molecule 1 (CD106): A multifaceted regulator of joint inflammation. Arthritis Rheum 44:985–994. https://doi.org/10.1002/1529-0131(200105)44:5<985::AID-ANR176>3.0.CO;2-P
Park M, Kulkarni A, Beatty A et al (2015) Soluble endothelial cell selective adhesion molecule and cardiovascular outcomes in patients with stable coronary disease: A report from the Heart and Soul Study. Atherosclerosis 243:546–552. https://doi.org/10.1016/j.atherosclerosis.2015.10.092
Yuri Gasparyan A, Ayvazyan L, Cocco G, Kitas DG (2012) Adverse Cardiovascular Effects of Antirheumatic Drugs: Implications for Clinical Practice and Research. Curr Pharm Des 18:1543–1555. https://doi.org/10.2174/138161212799504759
Disclosure
The authors confirm that they have no conflicts of interest related to this research.
Author information
Authors and Affiliations
Contributions
All authors of this manuscript have made significant contributions to the conception and design of the study, the acquisition and analysis of data, and the interpretation of results. They have all participated in the drafting and critical revision of the manuscript, and have given their final approval for submission to this journal. Additionally, all authors agree to be responsible for all aspects of the work and are willing to be held accountable for any issues that may arise. Finally, all contributing authors have reviewed and approved the final version of the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of the Topical Collection entitled ‘Cardiovascular Issues in Rheumatic Diseases’
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gerganov, G., Georgiev, T., Dimova, M. et al. Vascular effects of biologic and targeted synthetic antirheumatic drugs approved for rheumatoid arthritis: a systematic review. Clin Rheumatol 42, 2651–2676 (2023). https://doi.org/10.1007/s10067-023-06587-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06587-8