Abstract
Objective
There are few real-world setting studies focused on apremilast effectiveness (i.e., retention rate) in psoriatic arthritis (PsA). The main aim of this retrospective observational study is the assessment of apremilast 3-year retention rate in real-world PsA patients. Moreover, the secondary objective is to report the reasons of apremilast discontinuation and the factors related to treatment persistence.
Methods
In fifteen Italian rheumatological referral centers, all PsA consecutive patients who received apremilast were enrolled. Anamnestic data, treatment history, and PsA disease activity (DAPSA) at baseline were recorded. The Kaplan–Meier curve and the Cox analysis computed the apremilast retention rate and treatment persistence-related risk factors. A p-value < 0.05 was considered statistically significant.
Results
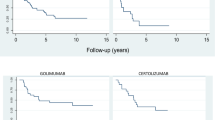
The 356 enrolled patients (median age 60 [interquartile range IQR 52–67] yrs; male prevalence 42.7%) median observation period was 17 [IQR 7–34] months (7218 patients-months). The apremilast retention rate at 12, 24, and 36 months was, respectively, 85.6%, 73.6%, and 61.8%. The main discontinuation reasons were secondary inefficacy (34% of interruptions), gastro-intestinal intolerance (24%), and primary inefficacy (19%). Age and oligo-articular phenotype were related to treatment persistence (respectively hazard ratio 0.98 IQR 0.96–0.99; p = 0.048 and 0.54 IQR 0.31–0.95; p = 0.03).
Conclusion
Almost three-fifths of PsA patients receiving apremilast were still in treatment after 3 years. This study confirmed its effectiveness and safety profile. Apremilast appears as a good treatment choice in all oligo-articular PsA patients and in those ones burdened by relevant comorbidities.
Key Points • Apremilast retention rates in this real-life cohort and trials are comparable. • The oligo-articular phenotype is associated with long-lasting treatment (i.e., 3 years). • No different or more prevalent adverse events were observed. |


Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Taylor W, Gladman D, Helliwell P et al (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54:2665–2673
Gossec L, Baraliakos X, Kerschbaumer A et al (2020) EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 79:700–712
Kavanaugh A, Mease PJ, Gómez-Reino JJ et al (2014) Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 73:1020–1026
Cutolo M, Myerson GE, Fleischmann RM et al (2016) A Phase III, Randomized, Controlled Trial of Apremilast in Patients with Psoriatic Arthritis: Results of the PALACE 2 Trial. J Rheumatol 43:1724–1734
Edwards CJ, Blanco FJ, Crowley J et al (2016) Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 75:1065–1073
Mease PJ, Gladman DD, Gómez-Reino JJ et al (2020) Long-Term Safety and Tolerability of Apremilast Versus Placebo in Psoriatic Arthritis: A Pooled Safety Analysis of Three Phase III, Randomized, Controlled Trials. ACR Open Rheumatol 2:459–470
Abignano G, Fadl N, Merashli M et al (2018) Apremilast for the treatment of active psoriatic arthritis: a single-centre real-life experience. Rheumatology (Oxford) 57:578–580
Favalli EG, Conti F, Atzeni F et al (2018) Comments on “Short-term reasons for withdrawal and adverse events associated with apremilast therapy for psoriasis in real-world practice compared with in clinical trials: A multicenter retrospective study.” J Am Acad Dermatol 79:e119–e120
Favalli EG, Conti F, Selmi C et al (2020) Retrospective evaluation of patient profiling and effectiveness of apremilast in an Italian multicentric cohort of psoriatic arthritis patients. Clin Exp Rheumatol 38:19–26
Schoels M, Aletaha D, Funovits J et al (2010) Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 69:1441–1447
Healy PJ, Helliwell PS (2008) Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 59:686–691
Porta S, Otero-Losada M, Kölliker-Frers RA et al (2020) Adipokines, Cardiovascular Risk, and Therapeutic Management in Obesity and Psoriatic Arthritis. Front Immunol 11:590749
Klingberg E, Bilberg A, Björkman S et al (2019) Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther 21:17–10
Papadavid E, Rompoti N, Theodoropoulos K et al (2018) Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol 32:1173–1179
Wong TH, Sinclair S, Smith B et al (2017) Real-world, single-centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol 42:675–676
Gladman DD, Ye JY, Chandran V et al (2021) Oligoarticular vs Polyarticular Psoriatic Arthritis: A Longitudinal Study Showing Similar Characteristics. J Rheumatol 48:1824–1829
Stekhoven D, Scherer A, Nissen MJ et al (2017) Hypothesis-free analyses from a large psoriatic arthritis cohort support merger to consolidated peripheral arthritis definition without subtyping. Clin Rheumatol 36:2035–2043
Mekhail C, Chouk M, Prati C et al (2020) Prognostic factors of good response to DMARDs in psoriatic arthritis: a narrative review. Expert Rev Clin Pharmacol 13:505–519
Schett G, Wollenhaupt J, Papp K et al (2012) Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 64:3156–3167
Ogdie A, Liu M, Glynn M et al (2021) Descriptive Comparisons of the Effect of Apremilast and Methotrexate Monotherapy in Oligoarticular Psoriatic Arthritis: The Corrona Psoriatic Arthritis/Spondyloarthritis Registry Results. J Rheumatol 48:693–697
Lucchetti R, Ceccarelli F, Cipriano E et al (2021) Application of Ultrasound in the Assessment of Oligoarticular Psoriatic Arthritis Subset: Results from Patients Treated with Apremilast. Isr Med Assoc J 23:412–415
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A Ariani has received honoraria as a speaker and an advisory board member of Amgen, Bristol-Myers Squibb, Boeringher, Bruno Farmaceutici, Janssen, Lilly, Novartis, Novo Nordisk, Sanofi, and Zentiva.
F Lumetti has received honoraria as an advisory board member of Amgen.
None of the other authors has any potential conflicts of interest to disclose in relation to this work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ariani, A., Parisi, S., Del Medico, P. et al. Apremilast retention rate in clinical practice: observations from an Italian multi-center study. Clin Rheumatol 41, 3219–3225 (2022). https://doi.org/10.1007/s10067-022-06255-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06255-3