Abstract
Background
Biologic disease-modifying antirheumatic drugs (bDMARDs) are recommended to be added in sequentially in the treatment of moderate-to-severe rheumatoid arthritis (RA). All these drugs, however, are substantially more expensive than conventional synthetic DMARDs throughout the world, including in China. The objective of this study is to evaluate the cost-effectiveness of treatment sequences of bDMARDs for patients with moderate-to-severe rheumatoid arthritis from the Chinese healthcare system perspective.
Methods
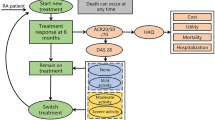
An individual patient simulation model was used to track the course of patients from first treatment through switches to further lines in a sequence. The comparator treatment sequence commenced with methotrexate, followed by non-biologic therapy. The intervention sequences were assumed to be the combinations of bDMARDs available, followed by non-biologic therapy. Life-years, quality-adjusted life-years (QALYs), and lifetime costs were estimated. Univariable and probabilistic sensitivity analyses and scenario analyses were performed to evaluate the model uncertainty.
Results
Compared with the comparator treatment sequence, bDMARDs sequences were associated with more life years, QALYs, and cost. These produced ICERs ranged from $27,441.36/QALY to $40,149.2/QALY, above the willingness-to-pay threshold of $10,378 per QALY. The uncertainty analyses and the scenario analyses confirmed the result of the base case analysis.
Conclusions
From the perspective of the Chinese healthcare system, bDMARDs sequences are estimated not to be cost-effective compared with conventional synthetic disease-modifying antirheumatic drug strategy for patients with moderate-to-severe RA at a WTP threshold of $10,378 per QALY. Price reductions are warranted to make bDMARDs cost-effective and affordable.



Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Coding is available on reasonable request.
References
Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z et al (2017) Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390(10093):457–468. https://doi.org/10.1016/S0140-6736(17)31618-5
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055):2023–2038. https://doi.org/10.1016/S0140-6736(16)30173-8
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T et al (2014) The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73(7):1316–1322. https://doi.org/10.1136/annrheumdis-2013-204627
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. https://doi.org/10.1016/S0140-6736(10)60826-4
Li R, Sun J, Ren LM, Wang HY, Liu WH, Zhang XW et al (2012) Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology (Oxford) 51(4):721–729. https://doi.org/10.1093/rheumatology/ker370
Documentation of the Second China National Samples Survey on Disability. China Statistics Press; 2007.
Lau CS, Chia F, Harrison A, Hsieh TY, Jain R, Jung SM et al (2015) APLAR rheumatoid arthritis treatment recommendations. Int J Rheum Dis 18(7):685–713. https://doi.org/10.1111/1756-185X.12754
Chinese RA (2018) 2018 Chinese guideline for the diagnosis and treatment of rheumatoid arthritis. Zhonghua Nei Ke Za Zhi 57(4):242–251. https://doi.org/10.3760/cma.j.issn.0578-1426.2018.04.004
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC et al (2016) 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 68(1):1–26. https://doi.org/10.1002/art.39480
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A et al (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79(6):685–699. https://doi.org/10.1136/annrheumdis-2019-216655
Ghabri S, Binard A, Pers YM, Maunoury F, Caro JJ (2020) Economic evaluation of sequences of biological treatments for patients with moderate-to-severe rheumatoid arthritis and inadequate response or intolerance to methotrexate in France. Value Health 23(4):461–470. https://doi.org/10.1016/j.jval.2019.12.003
Zhang X, Mu R, Wang X, Xu C, Duan T, An Y et al (2015) The impact of rheumatoid arthritis on work capacity in Chinese patients: a cross-sectional study. Rheumatology (Oxford) 54(8):1478–1487. https://doi.org/10.1093/rheumatology/kev014
Tan C, Li S, Yi L, Zeng X, Peng L, Qin S et al (2021) Tofacitinib in the treatment of moderate-to-severe rheumatoid arthritis in China: a cost-effectiveness analysis based on a mapping algorithm derived from a Chinese population. Adv Ther 38(5):2571–2585. https://doi.org/10.1007/s12325-021-01733-7
Incerti D, Curtis JR, Shafrin J, Lakdawalla DN, Jansen JP (2019) A flexible open-source decision model for value assessment of biologic treatment for rheumatoid arthritis. Pharmacoeconomics 37(6):829–843. https://doi.org/10.1007/s40273-018-00765-2
Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E et al (2013) Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 159(4):253–261. https://doi.org/10.7326/0003-4819-159-4-201308200-00006
Li ZG, Liu Y, Xu HJ, Chen ZW, Bao CD, Gu JR et al (2018) Efficacy and safety of tofacitinib in chinese patients with rheumatoid arthritis. Chin Med J (Engl) 131(22):2683–2692. https://doi.org/10.4103/0366-6999.245157
2015 report on Chinese nutrition and chronic disease. [database on the Internet]. The National Health and Family Planning Commission. Available from: http://www.gov.cn/xinwen/2015-06/30/content_2887030.htm
An Y, Liu T, He D, Wu L, Li J, Liu Y et al (2017) The usage of biological DMARDs and clinical remission of rheumatoid arthritis in China: a real-world large scale study. Clin Rheumatol 36(1):35–43. https://doi.org/10.1007/s10067-016-3424-5
Tian L, Xiong X, Guo Q, Chen Y, Wang L, Dong P et al (2020) Cost-effectiveness of tofacitinib for patients with moderate-to-severe rheumatoid arthritis in China. Pharmacoeconomics 38(12):1345–1358. https://doi.org/10.1007/s40273-020-00961-z
Carlson JJ, Ogale S, Dejonckheere F, Sullivan SD (2015) Economic evaluation of tocilizumab monotherapy compared to adalimumab monotherapy in the treatment of severe active rheumatoid arthritis. Value Health 18(2):173–179. https://doi.org/10.1016/j.jval.2014.10.013
Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K et al (2005) Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 7(4):R796-806. https://doi.org/10.1186/ar1740
Aletaha D, Smolen J (2005) The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 23(5 Suppl 39):S100–S108
Strand V, Miller P, Williams SA, Saunders K, Grant S, Kremer J (2017) Discontinuation of Biologic therapy in rheumatoid arthritis: analysis from the Corrona RA Registry. Rheumatol Ther 4(2):489–502. https://doi.org/10.1007/s40744-017-0078-y
Guyot P, Ades AE, Ouwens MJ, Welton NJ (2012) Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 12:9. https://doi.org/10.1186/1471-2288-12-9
Zhang J, Shan Y, Reed G, Kremer J, Greenberg JD, Baumgartner S et al (2011) Thresholds in disease activity for switching biologics in rheumatoid arthritis patients: experience from a large U.S. cohort. Arthritis Care Res (Hoboken) 63(12):1672–9. https://doi.org/10.1002/acr.20643
Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J et al (2016) Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess 20(35):1–610. https://doi.org/10.3310/hta20350
Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK et al (2011) Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011(2):CD008794. https://doi.org/10.1002/14651858.CD008794.pub2
Tosh J, Brennan A, Wailoo A, Bansback N (2011) The Sheffield rheumatoid arthritis health economic model. Rheumatology (Oxford). 2011;50 Suppl 4:iv26–31. https://doi.org/10.1093/rheumatology/ker243
Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F (2008) Biologic drugs for rheumatoid arthritis in the Medicare program: a cost-effectiveness analysis. Arthritis Rheum 58(4):939–946. https://doi.org/10.1002/art.23374
Wolfe F, Michaud K (2010) The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study. Arthritis Res Ther 12(2):R35. https://doi.org/10.1186/ar2944
Michaud K, Wallenstein G, Wolfe F (2011) Treatment and nontreatment predictors of health assessment questionnaire disability progression in rheumatoid arthritis: a longitudinal study of 18,485 patients. Arthritis Care Res (Hoboken) 63(3):366–372. https://doi.org/10.1002/acr.20405
Life tables for WHO member states (2016) World Health Organization, Geneva
Wolfe F, Michaud K, Gefeller O, Choi HK (2003) Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum 48(6):1530–1542. https://doi.org/10.1002/art.11024
Michaud K, Vera-Llonch M, Oster G (2012) Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol 39(1):54–59. https://doi.org/10.3899/jrheum.110491
Wu B, Wilson A, Wang FF, Wang SL, Wallace DJ, Weisman MH et al (2012) Cost effectiveness of different treatment strategies in the treatment of patients with moderate to severe rheumatoid arthritis in China. PLoS ONE 7(10):e47373. https://doi.org/10.1371/journal.pone.0047373
Health care and personal articles of consumer price indices. In: indices Hcapaocp, editor.: Health care and personal articles of consumer price indices.
Tanno M, Nakamura I, Ito K, Tanaka H, Ohta H, Kobayashi M et al (2006) Modeling and cost-effectiveness analysis of etanercept in adults with rheumatoid arthritis in Japan: a preliminary analysis. Mod Rheumatol 16(2):77–84. https://doi.org/10.1007/s10165-006-0461-y
China statistical yearbook 2020. National Bureau of Statistics of China.
Jansen JP, Incerti D, Mutebi A, Peneva D, MacEwan JP, Stolshek B et al (2017) Cost-effectiveness of sequenced treatment of rheumatoid arthritis with targeted immune modulators. J Med Econ 20(7):703–714. https://doi.org/10.1080/13696998.2017.1307205
Djalalov S, Beca J, Hoch JS, Krahn M, Tsao MS, Cutz JC et al (2014) Cost effectiveness of EML4-ALK fusion testing and first-line crizotinib treatment for patients with advanced ALK-positive non-small-cell lung cancer. J Clin Oncol 32(10):1012–1019. https://doi.org/10.1200/JCO.2013.53.1186
Bae YH, Mullins CD (2014) Do value thresholds for oncology drugs differ from nononcology drugs? J Manag Care Spec Pharm 20(11):1086–92. https://doi.org/10.18553/jmcp.2014.20.11.1086
Sarfaty M, Leshno M, Gordon N, Moore A, Neiman V, Rosenbaum E et al (2018) Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol 73(4):628–634. https://doi.org/10.1016/j.eururo.2017.07.041
China Guidelines for Pharmacoeconomic Evaluations (2020). Chinese Pharmaceutical Association; 2020.
Wan X, Zhang Y, Ma J, Tan C, Zeng X, Peng L (2019) Ribociclib in hormone-receptor-positive advanced breast cancer: establishing a value-based cost in China. Breast 43:1–6. https://doi.org/10.1016/j.breast.2018.10.004
Wan XM, Peng LB, Ma JA, Li YJ (2017) Economic evaluation of nivolumab as a second-line treatment for advanced renal cell carcinoma from US and Chinese perspectives. Cancer 123(14):2634–2641. https://doi.org/10.1002/cncr.30666
Wu B, Lu S (2020) The effect of PD-L1 categories-directed pembrolizumab plus chemotherapy for newly diagnosed metastatic non-small-cell lung cancer: a cost-effectiveness analysis. Transl Lung Cancer Res 9(5):1770–84. https://doi.org/10.21037/tlcr-19-605
Stevenson MD, Wailoo AJ, Tosh JC, Hernandez-Alava M, Gibson LA, Stevens JW et al (2017) The cost-effectiveness of sequences of biological disease-modifying antirheumatic drug treatment in England for patients with rheumatoid arthritis who can tolerate methotrexate. J Rheumatol 44(7):973–980. https://doi.org/10.3899/jrheum.160941
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 71874209) and Hunan Provincial Natural Science Foundation of China (No. 2019JJ40411). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
From the perspective of the Chinese healthcare system, biologic disease-modifying antirheumatic drug sequences are estimated not to be cost-effective compared with conventional synthetic disease-modifying antirheumatic drug strategy for patients with moderate-to-severe rheumatoid arthritis. Price reductions are warranted to make the biologic agents cost-effective and affordable.
Tan Chongqing and Luo Xia contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, C., Luo, X., Li, S. et al. Sequences of biological treatments for patients with moderate-to-severe rheumatoid arthritis in the era of treat-to-target in China: a cost-effectiveness analysis. Clin Rheumatol 41, 63–73 (2022). https://doi.org/10.1007/s10067-021-05876-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05876-4