Abstract
Introduction
The relationship between tophaceous gout and metabolic markers is not well understood. The aim of this study was to compare the correlations between different metabolic markers and tophi and evaluate their potential predictive values for tophus.
Method
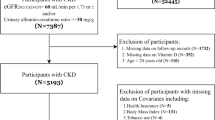
We analysed the data of gout patients in Beijing Jishuitan Hospital from 2013 to 2020. Ten laboratory indicators (UA, eGFR, underexcretion, GLU, TRIG, HDL-C, ALT, TBIL, γ-GT and UPH) were included to evaluate the relationship between tophaceous gout and metabolic markers.
Results
Tophi was present in 14.7% (119/808) of gout patients. UA, eGFR, ALT and γ-GT were independently related to the development of tophi; UA and γ-GT were positively correlated. The γ-GT/ALT ratio and UA/eGFR ratio showed a positive correlation with tophi, with (rho, P) of (0.305, < 0.001) and (0.195, < 0.001), respectively. The γ-GT/ALT ratio showed the best classificatory performance (AUC = 0.749, P < 0.001) for tophi among the four positive correlation indicators. With increasing integer γ-GT/ALT ratio, the incidence of tophi (4.9%, 9.7%, 22.3% and 38.4%, P < 0.001), chronic kidney disease (2.5%, 5.2%, 12.3% and 19.2%, P < 0.001) and hyperuricemia over 10 years (6.6%, 10.7%, 18.5% and 26.4%, P < 0.001) showed a progressive increase. The γ-GT/ALT ratio was positively correlated with the number of tophi and duration of hyperuricemia, negatively correlated with eGFR.
Conclusions
UA, eGFR, γ-GT and ALT were independently associated with tophi. The γ-GT/ALT ratio may be used as a predictor or monitor of tophi.
Key Points • We found that UA, eGFR, γ-GT and ALT were independently related to tophi among clinical commonly indicators. • The γ-GT/ALT ratio performed best in the identification of tophaceous gout from gout. • The incidence of tophi and chronic kidney disease and duration of hyperuricemia increased with γ-GT/ALT ratio. |




Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Danve A, Neogi T (2020) Rising global burden of gout: time to act. Arthritis Rheumatol 72:1786–1788. https://doi.org/10.1002/art.41453
Khanna P, Johnson RJ, Marder B, LaMoreaux B, Kumar A (2020) Systemic urate deposition: an unrecognized complication of gout? J Clin Med 9.https://doi.org/10.3390/jcm9103204
Thottam GE, Krasnokutsky S, Pillinger MH (2017) Gout and metabolic syndrome: a tangled web. Curr Rheumatol Rep 19:60. https://doi.org/10.1007/s11926-017-0688-y
Eslam M, Newsome PN, Sarin SK et al (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73:202–209. https://doi.org/10.1016/j.jhep.2020.03.039
Kuo CF, Yu KH, Luo SF et al (2010) Gout and risk of non-alcoholic fatty liver disease. Scand J Rheumatol 39:466–471. https://doi.org/10.3109/03009741003742797
Perez-Ruiz F, Martínez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E (2014) Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis 73:177–182. https://doi.org/10.1136/annrheumdis-2012-202421
Amanzada A, Goralczyk AD, Schneider S et al (2012) High predictability of a sustained virological response (87%) in chronic hepatitis C virus genotype 1 infection treatment by combined IL28B genotype analysis and γ-glutamyltransferase/alanine aminotransferase ratio: a retrospective single-center study. Digestion 86:218–227. https://doi.org/10.1159/000339879
Neogi T, Jansen TL, Dalbeth N et al (2015) 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 74:1789–1798. https://doi.org/10.1136/annrheumdis-2015-208237
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Lu B, Lu Q, Huang B, Li C, Zheng F, Wang P (2020) Risk factors of ultrasound-detected tophi in patients with gout. Clin Rheumatol 39:1953–1960. https://doi.org/10.1007/s10067-020-04947-2
Singh JA, Gaffo A (2020) Gout epidemiology and comorbidities. Semin Arthritis Rheum 50:S11-s16. https://doi.org/10.1016/j.semarthrit.2020.04.008
Battelli MG, Polito L, Bolognesi A (2014) Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis 237:562–567. https://doi.org/10.1016/j.atherosclerosis.2014.10.006
Jung JH, Song GG, Ji JD et al (2018) Metabolic syndrome: prevalence and risk factors in Korean gout patients. Korean J Intern Med 33:815–822. https://doi.org/10.3904/kjim.2016.062
Bolzetta F, Veronese N, Manzato E, Sergi G (2012) Tophaceous gout in the elderly: a clinical case review. Clin Rheumatol 31:1127–1132. https://doi.org/10.1007/s10067-012-1956-x
Dalbeth N, Jones G, Terkeltaub R et al (2019) Efficacy and safety during extended treatment of lesinurad in combination with febuxostat in patients with tophaceous gout: CRYSTAL extension study. Arthritis Res Ther 21:8. https://doi.org/10.1186/s13075-018-1788-4
Ma L, Sun R, Jia Z et al (2018) Clinical characteristics associated with subcutaneous tophi formation in Chinese gout patients: a retrospective study. Clin Rheumatol 37:1359–1365. https://doi.org/10.1007/s10067-017-3969-y
Corti A, Belcastro E, Dominici S, Maellaro E, Pompella A (2020) The dark side of gamma-glutamyltransferase (GGT): pathogenic effects of an “antioxidant” enzyme. Free Radic Biol Med 160:807–819. https://doi.org/10.1016/j.freeradbiomed.2020.09.005
Lee MY, Hyon DS, Huh JH et al (2019) Association between serum gamma-glutamyltransferase and prevalence of metabolic syndrome using data from the Korean Genome and Epidemiology Study. Endocrinol Metab (Seoul) 34:390–397. https://doi.org/10.3803/EnM.2019.34.4.390
Wang G, Lu X, Du Q et al (2020) Diagnostic value of the γ-glutamyltransferase and alanine transaminase ratio, alpha-fetoprotein, and protein induced by vitamin K absence or antagonist II in hepatitis B virus-related hepatocellular carcinoma. Sci Rep 10:13519. https://doi.org/10.1038/s41598-020-70241-5
Liu J, Xu C, Ying L et al (2017) Relationship of serum uric acid level with non-alcoholic fatty liver disease and its inflammation progression in non-obese adults. Hepatol Res 47:E104-e112. https://doi.org/10.1111/hepr.12734
Shen ZW, Xing J, Wang QL et al (2017) Association between serum γ-glutamyltransferase and chronic kidney disease in urban Han Chinese: a prospective cohort study. Int Urol Nephrol 49:303–312. https://doi.org/10.1007/s11255-016-1429-2
Sette LH, Lopes EP (2015) The reduction of serum aminotransferase levels is proportional to the decline of the glomerular filtration rate in patients with chronic kidney disease. Clinics (Sao Paulo) 70:346–349. https://doi.org/10.6061/clinics/2015(05)07
Author information
Authors and Affiliations
Contributions
Wei Liu, Hui Song, Siliang Man, Hongchao Li and Siming Gao carried out the studies, participated in collecting data and drafted the manuscript. Wei Liu, Hui Song and Siliang Man performed the statistical analysis and participated in its design. Wei Liu, Hongchao Li and Siming Gao helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This project was approved by the Ethics Committee of Beijing Jishuitan Hospital (No. 202008–01).
Consent to participate
The informed consent was waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, W., Song, H., Man, S. et al. Simple metabolic markers associated with tophaceous gout. Clin Rheumatol 40, 5047–5053 (2021). https://doi.org/10.1007/s10067-021-05861-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05861-x