Abstract
Introduction/objectives
To investigate the association between serum biomarker [cartilage oligomeric matrix protein (COMP) and matrix metalloproteinase-3 (MMP-3)] levels and clinical, magnetic resonance imaging (MRI), and arthroscopic findings in anterior cruciate ligament (ACL)-deficient knees without osteoarthritic changes on radiographs.
Method
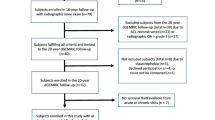
Patients with ACL injury of Kellgren–Lawrence grade 0 or 1 were enrolled. Serum COMP and MMP-3 levels were measured preoperatively. Correlations of serum biomarker levels with age, body mass index (BMI), duration from time of injury, Tegner activity scale (TAS) score, Lysholm knee score, International Knee Documentation Committee score, KT-1000 arthrometer measurements, whole-organ MRI score (WORMS), MRI T2 relaxation time, and arthroscopic International Cartilage Research Society (ICRS) grade were assessed by calculating Spearman correlation coefficients. Associations between intraoperative findings (cartilage, meniscus) and serum biomarker levels were determined using the Mann–Whitney U test. Multiple regression analysis was performed to investigate the correlations between serum biomarker levels and MRI and arthroscopic findings.
Results
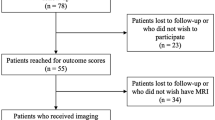
Ninety-eight patients with a mean age of 23.7 years were enrolled. Higher serum COMP level was correlated with older age and higher BMI, TAS score, serum MMP-3 level, WORMS, and T2 relaxation times (medial femur, medial tibia). Multivariate analysis showed that the serum COMP level was independently associated with WORMS and ICRS grade.
Conclusions
The serum COMP level was correlated with age, BMI, TAS score, and MMP-3 level in ACL-deficient knees and was independently correlated with WORMS and ICRS grade. Thus, the serum COMP level can help detect cartilage degeneration even in patients without radiographic osteoarthritic changes.
Key Points • Serum COMP correlated with WORMS and ICRS grade in ACL deficient knee. • The serum COMP level could help in detecting cartilage degeneration, even in patients with no radiographic osteoarthritic changes. |


Similar content being viewed by others
Data availability
The registered data is managed by the Ethical Review Committee of this hospital.
References
Carbone A, Rodeo S (2017) Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res 35:397–405
Thomas AC, Hubbard-Tumer T, Wilkstrom EA, Palmieri-Smith RM (2017) Epidemiology of posttraumatic osteoarthritis. J Athl Train 52:491–496
Marijnissen AC, Vincken KL, Vos PA, Saris DB, Viergever MA, Bijlsma JW, Bartels LW, Lafeber FP (2008) Knee Images Digital Analysis (KIDA): a novel method to quantify individual radiographic features of knee osteoarthritis in detail. Osteoarthritis Cartilage 16:234–243
Chu CR, Williams AA, Coyle CH, Bowers ME (2012) Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther 14:212
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Eckstein F, Burstein D, Link TM (2006) Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed 19:822–854
Luyten FP, Denti M, Filardo G, Kon E, Engebretsen L (2012) Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20:401–406
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D et al (2004) Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12:177–190
Liess C, Lusse S, Karger N, Heller M, Gluer CC (2002) Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage 10:907–913
Mlynarik V, Trattnig S, Huber M, Zembsch A, Imhof H (1999) The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging 10:497–502
Xia Y (2000) Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol 35:602–621
Shruti M, Gaurav P, Sapna S, Radhika B (2019) T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol J Radiol 84:e549–e564
Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, Kraus VB (1999) Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County osteoarthritis project. Arthritis Rheum 42:2356–2364
Tseng S, Reddi AH, Di Cesare PE (2009) Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark Insights 4:33–44
Verma P, Dalal K (2013) Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J Orthop Res 31:999–1006
Hosnijeh FS, Runhaar J, Meurs JBJ, Bierma-Zeinstra SM (2015) Biomarkers for osteoarthritis: can they be used for risk assessment? A systematic review. Maturitas 82:36–49
Palmieri-Smith RM, Wojtys EM, Potter HG (2016) Early cartilage changes after anterior cruciate ligament injury: evaluation with imaging and serum biomarkers—a pilot study. Arthroscopy 32:1309–1318
Georgiev T, Ivanova M, Kopchev A, Velikova T, Miloshov A, Kurteva E, Yuzeir K, Penkov M, Kabakchieva P, Rashkov R, Stoilov R (2018) Cartilage oligomeric protein, matrix metalloproteinase-3, and Coll2-1 as serum biomarkers in knee osteoarthritis: a cross-sectional study. Rheumatol Int 38:821–830
Joseph GB, Nevitt MC, McCulloch CE, Neumann J, Lynch JA, Heilmeier U et al (2018) Associations between molecular biomarkers and MR-based cartilage composition and knee joint morphology: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 26:1070–1077
Jiao Q, Wei L, Chen C, Li P, Wang X, Li Y, Guo L, Zhang C, Wei X (2016) Cartilage oligomeric matrix protein and hyaluronic acid are sensitive serum biomarkers for early cartilage lesions in the knee joint. Biomarkers 21:146–151
Ma JD, Zhou JJ, Zheng DH, Chen LF, Mo YQ, Wei XN et al (2014) Serum matrix metalloproteinase-3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis. Mediators Inflamm 2014:179284
Deveza LA, Kraus VB, Collins JE, Guermazi A, Roemer FW, Nevitt MC, Hunter DJ (2018) Is synovitis detected on non-contrast-enhanced magnetic resonance imaging associated with serum biomarkers and clinical signs of effusion? Data from the Osteoarthritis Initiative. Scand J Rheumatol 47:235–242
Li W, Du C, Wang H, Zhang C (2014) Increased serum ADAMTS-4 in knee osteoarthritis: a potential indicator for the diagnosis of osteoarthritis in early stages. Genet Mol Res 13:9642–9649
Pengas I, Eldridge S, Assiotis A, McNicholas M, Mendes JE, Laver L (2018) MMP-3 in the peripheral serum as a biomarker of knee osteoarthritis, 40 years following open total knee meniscectomy. J Exp Orthop 5:21
Favero M, Belluzzi E, Trisolino G, Goldring MB, Goldring SR, Cigolotti A, Pozzuoli A, Ruggieri P, Ramonda R, Grigolo B, Punzi L, Olivotto E (2019) Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: a coculture study. J Cell Physiol 234:11176–11187
Risberg MA, Oiestad BE, Gunderson R, Aune AK, Engebretsen L, Culvenor A, Holm I (2016) Changes in knee osteoarthritis, symptoms, and function after anterior cruciate ligament reconstruction: a 20-year prospective follow-up study. Am J Sports Med 44:1215–1224
Keays SL, Newcombe PA, Bullock-Saxton JE, Bullock MI, Keays AC (2010) Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med 38:455–463
Mundermann A, Dyrby CO, Andriacchi TP, King KB (2005) Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthritis Cartilage 13:34–38
Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R (1985) Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg Am 67:720–726
Tao H, Qiao Y, Hu Y, Xie Y, Lu R, Yan X, Chen S (2018) Quantitative T2-mapping and T2 *-mapping evaluation of changes in cartilage matrix after acute anterior cruciate ligament rupture and the correlation between the results of both methods. Biomed Res Int 17:7985672–7985678. https://doi.org/10.1155/2018/7985672
Gong J, Pedoia V, Facchetti L, Link TM, Ma CB, Li X (2016) Bone marrow edema-like lesions (BMELs) are associated with higher T1ρ and T2 values of cartilage in anterior cruciate ligament (ACL)-reconstructed knees: a longitudinal study. Quant Imaging Med Surg 6:661–670
Bolbos RI, Link TM, Ma CB, Majumdar S, Li X (2009) T1ρ relaxation time of the meniscus and its relationship with T1ρ of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis Cartilage 17:12–18
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A:58–69
Luc B, Gribble PA, Pietrosimone BG (2014) Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers needed-to-treat analysis. J Athl Train 49:806–819
Lohmander LS, Ostenberg A, Englund M, Roos H (2004) High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum 50:3145–3152
Jones MH, Spindler KP (2017) Risk factors for radiographic joint space narrowing and patient reported outcomes of post-traumatic osteoarthritis after ACL reconstruction: data from the MOON cohort. J Orthop Res 35:1366–1374
Andriacchi TP, Dyrby CO (2005) Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech 38:293–298
Koelling S, Clauditz TS, Kaste M, Miosge N (2006) Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res Ther 8:R56
Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ (2004) Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biolumin Chemilumin 279:19502–19511
Ribbens C, Porras MM, Franchimont N, Kaiser MJ, Jaspar JM, Damas P et al (2002) Increased matrix metalloproteinase-3 serum levels in rheumatic diseases: relationship with synovitis and steroid treatment. Ann Rheum Dis 61:161–166
Kaplan DJ, Cuellar VG, Jazrawi LM, Strauss E (2017) Biomarker changes in anterior cruciate ligament-deficient knees compared with healthy controls. Arthroscopy 33:1053–1061
Pelletier JP, Raynauld JP, Caron J, Mineau F, Abram F, Dorais M, Haraoui B, Choquette D, Martel-Pelletier J (2010) Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann Rheum Dis 69:2095–2101
Jungmann PM, Baum T, Nevitt MC, Nardo L, Gersing AS, Lane NE, McCulloch CE, Rummeny EJ, Link TM (2016) Degeneration in ACL injured knees with and without reconstruction in relation to muscle size and fat content-data from the osteoarthritis initiative. PLoS One 11:e0166865
Snoj Z, Zupanc O, Salapura V (2016) Retrospective quantitative cartilage and semi-quantitative morphological evaluation at 6 years after ACL reconstruction. Arch Orthop Trauma Surg 136:967–974
Keene GC, Bickerstaff D, Rae PJ, Paterson RS (1993) The natural history of meniscal tears in anterior cruciate ligament insufficiency. Am J Sports Med 21:672–679
Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S (2004) A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng 32:447–457
Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, Moretti S, Munegato D, Augusti CA, Bresciani E, Omeljaniuk RJ, Locatelli V, Torsello A (2013) Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res 31:315–321
Manicourt DH, Fujimoto N, Obata K, Thonar EJ (1994) Serum levels of collagenase, stromelysin-1, and TIMP-1. Age- and sex-related differences in normal subjects and relationship to the extent of joint involvement and serum levels of antigenic keratan sulfate in patients with osteoarthritis. Arthritis Rheum 37:1774–8173
Rodriguez KM, Curran MT, Garcia SA, Mendias CL, Palmieri-Smith RM (2020) Sex, but not body mass index, influences MMP-3 and type 2 collagen turnover following ACL injury and reconstruction. Osteoarthritis and Cartilage 28:S325
Acknowledgment
The authors thank the Japan Foundation for Aging and Health, and SRL Inc. for the biomarker analysis. They also thank Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the conception and/or design of the study (YN and YH), acquisition of data (YN, KO, KN, and TK), analysis and interpretation of data (YN and YH), and final approval of the article (YH, HN). All authors participated in the writing process and approved the final version of the manuscript. Yusuke Hashimoto (m1375526@@med.osaka-cu.ac.jp) takes responsibility for the integrity of the work.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the hospital ethics committee and an internal review board of our institution.
Consent to participate and consent for publication
Informed consent to participate and publication were obtained from all the enrolled patients.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishida, Y., Hashimoto, Y., Orita, K. et al. Serum cartilage oligomeric matrix protein is correlated with quantitative magnetic resonance imaging and arthroscopic cartilage findings in anterior cruciate ligament deficient knees without osteoarthritic changes. Clin Rheumatol 40, 4629–4638 (2021). https://doi.org/10.1007/s10067-021-05800-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05800-w