Abstract
Introduction
Anti-Ro/SSA and anti-La/SSB antibodies are associated with neonatal lupus and congenital heart block. Controversial results regarding perinatal outcomes are found and less is known about aneuploidy screening. The hypothesis is that the presence of anti-Ro and/or anti-La antibodies influences the levels of PAPP-A and ß-HCG, thus interfering in the calculation of risk of aneuploidies.
Material and methods
Fifty-five anti-Ro/SSA positive pregnant women were included. The demographic characteristics and laboratory variables were studied. Data concerning chromosomopaties screening were also recorded.
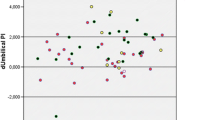
Results
PAPP-A and β-HCG levels were calculated (as well as NT and CRL) and compared with a healthy cohort of 12971 pregnant women. PAPP-A levels in mg/mL were lower significatively. In anti-La/SS-B cohort, significant differences were found in PAPP-A in mg/mL and in MoM. Combined risks for Down syndrome (DS) in both groups were higher but the differences were due to age.
Conclusions
Serum levels of PAPP-A were significative lower but not confirmed when adjusted to MoM. This will have to be confirmed in studies with a larger number of patients and to check whether there is an impact in the calculation of DS risk or not. They could represent a group of pregnant women with significantly a higher risk of adverse perinatal outcome.
Key Points • Pregnant patients with anti-Ro/SS-A ant/or anti-La/SS-B antibodies have low PAPP-A levels compared with pregnant women without antibodies. • PAPP-A levels are used in obstetrics for aneuploidies screening in the first trimester, so in these patients, there could be more false positive screening. • In these findings are verified in trials with a larger number of patients, a correction variable would have to be applied for the aneuploidies screening calculation. • Also, low PAPP-A levels are correlated with poor placentation, that is to say, more risk of miscarriages, small fetus for gestational age, and preeclampsia. This is another topic to take into consideration in this population. |
Similar content being viewed by others
References
Satoh M, Chan EK, Ho LA et al (2012) Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 64:2319–2327
Bielsa Masdeu AM (2010) Autoanticuerpos. Guía rápida, 2nd edn. Ana María Bielsa Masdeu, Madrid
Guo YP, Wang CG, Liu X, Huang YQ, Guo DL, Jing XZ, Yuan CG, Yang S, Liu JM, Han MS, Li HX (2014) The prevalence of antinuclear antibodies in the general population of china: a cross-sectional study. Curr Ther Res Clin Exp 76:116–119
Lee LA, Bias WB, Arnett FC Jr et al (1983) Immunogenetics of the neonatal lupus syndrome. Ann Intern Med 99:592–596
Watson RM, Lane AT, Barnett NK, Bias WB, Arnett FC, Provost TT (1984) Neonatal lupus erythematosus A clinical, serological and immunogenetic study with review of the literature. Medicine 63:362–378
Brito-Zerón P, Pasoto SG, Robles-Marhuenda A, Mandl T, Vissink A, Armagan B, Praprotnik S, Nocturne G, Sebastian A, Fernandes Moça Trevisani V, Retamozo S, Acar-Denizli N, Wiland P, Sisó-Almirall A, Bootsma H, Mariette X, Ramos-Casals M, Kostov B, Sjögren Big Data Consortium (2020) Autoimmune congenital heart block and primary Sjögren’s syndrome: characterisation and outcomes of 49 cases. Clin Exp Rheumatol 126(4):95–102
Hull RG, Harris EN, Morgan SH, Hughes GR (1983) Anti-Ro antibodies and abortions in women with SLE. Lancet 2:1138
Watson RM, Braunstein BL, Watson AJ, Hochberg MC, Provost TT (1986) Fetal wastage in women with anti-Ro(SSA) antibody. J Rheumatol 13:90–94
Mavragani CP, Dafni UG, Txioufas AG, Moutsopoulos HM (1998) Pregnancy outcome and anti-Ro/SS-A in autoimmune disease: a retrospective cohort study. Br J Rheumatol 37:740–745
Le Thi HD, Wechsler B, Piette JC, Bletry O, Godeau P (1994) Pregnancy and its outcome in systemic lupus erythematosus. Q J Med 87:721–729
Brucato A, Doria A, Frassi M, Castellino G, Franceschini F, Faden D, Pisoni MP, Solerte L, Muscarà M, Lojacono A, Motta M, Cavazzana I, Ghirardello A, Vescovi F, Tombini V, Cimaz R, Gambari PF, Meroni PL, Canesi B, Tincani A (2002) Pregnancy outcome in 100 women with autoimmune diseases and anti-Ro/SSA antibodies: a prospective controlled study. Lupus 11:716–721
Skog A, Lagnefeldt L, Conner P, Wahren-Herlenius M, Sonesson SE (2016) Outcome in 212 anti-Ro/SS-A positive pregnancies and population-based incidence of congenital heart block. Acta Obstet Gynecol Scand 95:98–105
Martínez-Sánchez N, Pérez-Pinto S, Robles-Marhuenda Á, Arnalich-Fernández F, Martín Cameán M, Hueso Zalvide E, Bartha JL (2017) Obstetric and perinatal outcome in anti-Ro/SSA-positive pregnant women: a prospective cohort study. Immunol Res 65:487–494. https://doi.org/10.1007/s12026-016-8888-5
Lisney AR, Szelinski F, Reiter K, Burmester GR, Rose T, Dorner T (2017) High maternal expression of SIGLEC1 on monocytes as a surrogate marker of a type I interferon signature is a risk factor for the development of autoimmune congenital heart block. Ann Rheum Dis 76:1476–1480
Clancy RM, Halushka M, Rasmussen SE, Lhakhang T, Chang M, Buyon JP (2019) Siglec-1 macrophages and the contribution of IFN to the development of autoimmune congenital heart block. J Immunol 1(202):48–55
Ambrosi A, Thorlacius GE, Sonesson SE, Wahren-Herlenius M (2021) Interferons and innate immune activation in autoimmune congenital heart block. Scand J Immunol 93(1):e12995. https://doi.org/10.1111/sji.12995
Nicolaides KH (2011) Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn 31:7–15
Nicolaides KH, Spencer K, Avgidou K, Faiola S, Falcon O (2005) Multicenter study of first-trimester screening for trisomy 21 in 75 821 pregnancies: results and estimation of the potential impact of individual risk-orientated two-stage first-trimester screening. Ultrasound Obstet Gynecol 25:221–226
Merkatz IR, Nitowsky HM, Macri JN, Johnson WE (1984) An association between low maternal serum alpha-fetoprotein and fetal chromosomal abnormalities. Am J Obstet Gynecol 148:886–894
Canick J, Knight GJ, Palomaki GE et al (1988) Low second trimester maternal serum unconjugated oestriol in pregnancies with Down’s syndrome. BJOG 95:330–333
Macri JN, Kasturi RV, Krantz DA, Cook EJ, Moore ND, Young JA, Romero K, Larsen JW Jr (1990) Maternal serum Down syndrome screening: free beta protein is a more effective marker than human chorionic gonadotrophin. Am J Obstet Gynecol 163:1248–1253
Van Lith JM, Pratt JJ, Beekhuis JR, Mantingh A (1993) Second trimester maternal serum immuno-reactive inhibin as a marker for fetal Down’s syndrome. Prenat Diagn 12:801–806
Brambati B, Macintosh MCM, Teisner B, Maguiness S, Shrimanker K, Lanzani A, Bonacchi I, Tului L, Chard T, Grudzinskas JG (1993) Low maternal serum level of pregnancy associated plasma protein (PAPP-A) in the first trimester in association with abnormal fetal karyotype. BJOG 100:324–326
Aitken DA, Wallace EM, Crossley JA, Swanston IA, van Pareren Y, van Maarle M, Groome NP, Macri JN, Connor JM (1996) Dimeric inhibin A as a marker for Down’s syndrome in early pregnancy. N Engl J Med 334:1231–1236
Spenser K, Cowans NJ (2013) Correction of first trimester biochemical aneuploidy screening markers for smoking status: influence of gestational age, maternal ethnicity and cigarette dosage. Prenat Diagn 33:11–123
Kagan KO, Wright D, Spencer K, Molina FS, Nicolaides KH (2008) First-trimester screening for trisomy 21 by free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A: impact of maternal and pregnancy characteristics. Ultrasound Obstet Gynecol 31:493–502
Spencer K, Cowans NJ, Spencer CE, Achillea N (2010) A re-evaluation of the influence of maternal insulin-dependent diabetes on fetal nuchal translucency thickness and first-trimester maternal serum biochemical markers of aneuploidy. Prenat Diagn 30:937–940
Kuc S, Wortelboer E, Koster M, De Valk H, Schielen P, Visser G (2011) Prediction of macrosomia at birth in type-1 and 2 diabetic pregnancies with biomarkers of early placentation. BJOG 118:748–754
Savvidou MD, Syngelaki A, Muhaisen M, Emelyanenko E, Nicolaides KH (2012) First trimester maternal serum free β-human chorionic gonadotropin and pregnancy-associated plasma protein A in pregnancies complicated by diabetes mellitus. BJOG 119(4):410–416
Madsen HN, Ekelund CK, Tørring N, Ovesen PG, Friis-Hansen L, Ringholm L et al (2012) Impact of type 1 diabetes and glycemic control on fetal aneuploidy biochemical markers. Acta Obstet Gynecol Scand 91:57–61
Lo YM et al (1997) Presence of fetal DNA in maternal plasma and serum. Lancet 350:485–487
Papageorgiou EA, Karagrigoriou A, Tsaliki E, Velissariou V, Carter NP, Patsalis PC (2011) Fetal-specific DNA methylation ratio permits noninvasive prenatal diagnosis of trisomy 21. Nat Med 17:510–513
Gil M et al (2017) Analysis of cell-free DNA in maternal blood screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 50:302–314
Clark F, Dickinson JE, Walters BN, Marshall LR, O’Leary PC (1995) Elevated mid-trimester hCG and maternal lupus anticoagulant. Prenat Diagn 15:1035–1039
Ferriman EL, Sehmi IK, Jones R, Railton A, Hilton RC, Cuckle HS (2000) False-positive maternal serum screening in systemic lupus erythematosis: a case report. Prenat Diagn 20:851
Maymon R, Sehmi IK, Herman A, Jones RC, Sherman D, Cuckle H (2000) Serum inhibin A levels in pregnant women with systemic lupus erythematosus or antiphospholipid syndrome. Prenat Diagn 20:12–16
Rego de Sousa MJ et al (2019) First trimester combined screening in patients with systemic lupus erythematosus: impact of pre-analytical variables on risk assessment. Clin Rheumatol 38(5):1251–1255
Yilmaz ZV, Türkmen GG, Yilmaz E, Daglar K, Kibas A, Sanhal C, Yücel A, Uygur D (2017) Influence of Behçet’s disease on first and second trimester serum screening markers. J Obstet Gynaecol Res 43(3):511–515
Spencer CA, Allen VM, Flowerdew G, Dooley K, Dodds L (2008) Low levels of maternal serum PAPP-A in early pregnancy and the risk of averses outcomes. Prenat Diagn 28(11):1029–1036. https://doi.org/10.1002/pd.2116
Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, Conover CA (1999) The insulin-like growth factor (IGF)-dependent binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci 96:3149–3153
Ong CYT, Liao AW, Spencer K, Munim S, Nicolaides KH (2000) First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. Br J Obstet Gynaecol 107:1265–1270
Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, Hankins G, Berkowitz RL, Merkatz I, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, Vidaver J, D'Alton ME (2004) First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (The FASTER Trial). Am J Obstet Gynecol 191:1446–1451
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martínez-Sánchez, N., Robles Marhuenda, A., De la Calle Fernández-Miranda, M. et al. First trimester combined screening test for aneuploidies in anti-Ro carriers pregnant women. Clin Rheumatol 40, 2699–2705 (2021). https://doi.org/10.1007/s10067-021-05616-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05616-8