Abstract
Objectives
To evaluate the ability of geldanamycin to modulate two opposing TNFα/TNFR1-triggered signals for inflammation and cell death.
Methods
The effects of geldanamycin on TNFα-induced proinflammatory cytokine production, apoptosis, NF-κB activation, caspase activation, and necroptosis in a human rheumatoid synovial cell line (MH7A) were evaluated via ELISA/qPCR, flow cytometry, dual-luciferase reporter assay, and western blotting assay, respectively. In addition, therapeutic effects on murine collagen-induced arthritis (CIA) were also evaluated.
Results
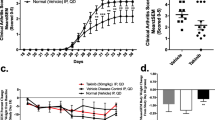
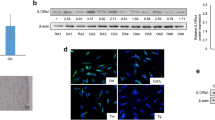
Geldanamycin disrupted RIPK1 in MH7A, thereby inhibiting TNFα-induced proinflammatory cytokine production and enhancing apoptosis. TNFα-induced NF-κB and MLKL activation was inhibited, whereas caspase 8 activation was enhanced. Recombinant RIPK1 restored the geldanamycin-mediated inhibition of TNFα-induced NF-κB activation. In addition, GM showed more clinical effectiveness than a conventional biologic TNF inhibitor, etanercept, in murine CIA and significantly attenuated synovial hyperplasia, a histopathological hallmark of RA.
Conclusions
GM disrupts RIPK1 and selectively inhibits the TNFR1-triggered NF-κB activation signaling pathway, while enhancing the apoptosis signaling pathway upon TNFα stimulation, thereby redressing the balance between these two opposing signals in a human rheumatoid synovial cell line. Therapeutic targeting RIPK1 may be a novel concept which involves TNF inhibitor acting as a TNFR1-signal modulator and have great potential for a more fundamental, effective, and safer TNF inhibitor.
Key Points • Geldanamycin (GM) disrupts RIPK1 and selectively inhibits the TNFR1-triggered NF-κB activation signaling pathway while enhancing the apoptosis signaling pathway upon TNFα stimulation, thereby redressing the balance between these two opposing signals in a human rheumatoid synovial cell line, MH7A. • GM showed more clinical effectiveness than a conventional biologic TNF-inhibitor, etanercept, in murine collagen-induced arthritis (CIA), and significantly attenuated synovial hyperplasia, a histopathological hallmark of RA. • Therapeutic targeting RIPK1 may be a novel concept which involves TNF inhibitor acting as a TNFR1-signal modulator and have great potential for a more fundamental, effective, and safer TNF-inhibitor. |






Similar content being viewed by others
Data availability
All data and materials are available with permission of the authors.
Abbreviations
- RIPK1:
-
Receptor-interacting serine/threonine-protein kinase 1
- GM:
-
Geldanamycin
- TNF:
-
Tumor necrosis factor
- TNFR:
-
TNFα receptor
- TNFi:
-
TNF-inhibitor
- IL-1β:
-
Interleukin-1beta
- IL-6:
-
Interleukin-6
- ELISA:
-
Enzyme-linked immunosorbent assay
- qPCR:
-
Quantitative real-time polymerase chain reaction
- RA:
-
Rheumatoid arthritis
- FLS:
-
Fibroblast-like synovial cells
- NF-κB:
-
Nuclear factor-kappa B
- HSP:
-
Heat shock protein
- MLKL:
-
Mixed lineage kinase domain-like
- CIA:
-
Collagen-induced arthritis
- ETN:
-
Etanercept
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Extracellular signal-regulated kinase
References
Feldmann M, Brennan FM, Foxwell BM, Maini RN (2001) The role of TNF alpha and IL-1 in rheumatoid arthritis. Curr Dir Autoimmun 3:188–199
Scott DL (2012) Biologics-based therapy for the treatment of rheumatoid arthritis. Clin Pharmacol Ther 91:30–43
Caporali R, Crepaldi G, Codullo V, Benaglio F, Monti S, Todoerti M et al (2018) 20 years of experience with tumor necrosis factor inhibitors: what have we learned? Rheumatology 57:vii5–vi10
Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana G, Bertolaccini L et al (2007) Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine. 86:242–245
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V (2006) Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 295:2275–2285
Chung ES (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 107:3133–3140
Silva-Fernandez L, Hyrich K (2014) Rheumatoid arthritis: when TNF inhibitors fail in RA – weighing up the options. Nat Rev Rheumatol 10:262–264
Wajant H, Pfizenmaier K, Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10:45–65
Vandenabeele P, Declercq W, Beyaert R, Fiers W (1995) Two tumor necrosis factor receptors: structure and function. Trends Cell Biol 5:392–399
Wajant H, Scheurich P (2011) TNFR1-induced activation of the classical NF-κB pathway. FEBS J 278:862–876
Micheau O, Tschopp J (2003) Induction of TNF receptor I mediated apoptosis via two sequential signaling complexes. Cell 114:181–190
Liu H, Pope RM (2003) The role of apoptosis in rheumatoid arthritis. Curr Opin Pharmacol 3:317–322
Ceponis A, Hietanen J, Tamulaitiene M, Partsch G, Patiala H, Konttinen YT (1999) A comparative quantitative morphometric study of cell apoptosis in synovial membranes in psoriatic, reactive, and rheumatoid arthritis. Rheumatology. 38:431–440
Bartok B, Firestein GS (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 233:233–255
Firestein GS (2009) Etiology and pathogenesis of rheumatoid arthritis. In: Firestein GS, Budd RC, Harris T, IB MI, Ruddy S, Sergent JS (eds) Elsevier 2009Kelly’s Textbook of Rheumatology, vol 8, pp 1035–1086
Hsu H, Huang J, Shu H-B, Shu H-B, Baichwal V, Goeddel DV (1996) TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4:387–396
Cuchet-Lourenco D, Eletto D, Wu C, Plagnol V, Papapietro O, Curtis J et al (2018) Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 361:810–813
Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M (2014) RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513:90–94
Takahashi N, Vereecke L, Bertrand L, Duprez L, Berger SB, Divert T et al (2014) RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 4(513):95–99
Schulle TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D et al (1988) Antibiotic radicicol to N-terminal domain of Hsp90 and shares important biologic activities with geldanamaycin. Cell Stress Chaperones 3:100–108
Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, Liu ZG (2000) Disruption of Hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-κB activation. J Biol Chem 275:10519–10526
Ting AT, Pimentel-Muinos FX, Seed B (1996) RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but Fas/APO-1-initiated apoptosis. EMBO J 15:6189–6196
Miyazawa K, Mori A, Okudaira H (1998) Establishment and characterization of a novel human rheumatoid fibroblast-like synoviocyte line, MH7A, immortalized with SV40 T antigen. J Biochem 124:1153–1162
Zhou W, Yuan J (2014) Necroptosis in health and diseases. Semin Cell Dev Biol 35:14–23
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227
Williams R, Feldmann M, Maini RN (1992) Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A 89:9784–9788
Sasai M, Saeki Y, Ohshima S, Nishioka K, Mima T, Tanaka T, Katada Y, Yoshizaki K, Suemura M, Kishimoto T (1999) Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheumatol 42:1635–1643
Nii T, Kuzuya K, Kabata D, Matsui T, Murata A, Ohya T et al (2019) Crosstalk between tumor necrosis factor-alpha signaling and aryl hydrocarbon receptor signaling in nuclear factor-kappa B activation: a possible molecular mechanism underlying the reduced efficacy of TNF-inhibitors in rheumatoid arthritis by smoking: a possible molecular mechanism underlying the reduced efficacy of TNF-inhibitors in rheumatoid arthritis by smoking. J Autoimmn 98:95–102
Mori L, Iselin S, De Libero G, Lesslauer W (1996) Attenuation of collagen-induced arthritis in 55-kDa TNF receptor type 1 (TNFR1)-IgG1-treated and TNFR1-deficient mice. J Immunol 57:3178–3182
Simmonds RE, Foxwell BM (2008) Signalling, inflammation and arthritis: NF-κB and its relevance to arthritis and inflammation. Rheumatology. 47:584–590
Asahara H, Asanuma M, Ogawa N, Nishibayashi S, Inoue H (1995) High DNA-binding activity of transcription factor NF-κB in synovial membranes of patients with rheumatoid arthritis. Biochem Mol Biol Int 37:827–833
Linkermann A, Green DR (2014) Necroptosis. N Engl J Med 370:455–465
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15:135–147
Supko JG, Hickman RL, Grever MR, Malspeis L (1995) Preclinical pharmacological evaluation of geldanamycin as an anti-tumor agent. Cancer Chemother Pharmacol 36:305–315
Russo CD, Polak PE, Mercado PR, Spagnolo A, Sharp A, Murphy P, Kamal A, Burrows FJ, Fritz LC, Feinstein DL (2006) The heat-shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin suppresses glial inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurochem 99:1351–1362
Chen Y, Zhu J, Zhu F, Dai BB, Song SJ, Wang ZQ et al (2018) Necrostatin-1 ameliorates adjuvant arthritis rat articular chondrocyte injury via inhibiting ASIC1a-mediated necroptosis. Biochem Biophys Res Commun 504:843–850
Jhun J, Lee SH, Kim S-Y, Ryu J, Kwon JY, Na HS et al (2019) Min. RIPK1 inhibition attenuates experimental autoimmune arthritis via suppression of osteoclastogenesis. J Trans Med 17:84. https://doi.org/10.1186/s12967-019-1809-3
Acknowledgments
We would like to thank Mitsubishi-Tanabe Pharmaceutical Co., Ltd. for discussions, KAC Co., Ltd. for helping mouse experiments, and Editage (www.editage.jp) for English language editing. We use MH7A cells with the MTA from KISSEI Pharmaceutical CO., Ltd..
Funding
This study was supported partly by the grant from Japan National Hospital Organization (H28-NHO (Immunological disorder diseases)-02).
Author information
Authors and Affiliations
Contributions
Y.O., E.O., C.U., and A. M. performed key experiments and analyzed the data. T.T., Y.H., and SO performed or contributed to specific experiments. J.M. performed statistical analysis. Y.S. conceptualized the study, designed and supervised the experiments, analyzed data, and wrote the manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Code availability
Not applicable.
Additional information
Statements
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. All animal experimental procedures were performed in KAC Co. Ltd. Japan with the approval of the KAC Institutional Animal Care and Use Committee (No.18-1021) and the Animal Research Ethics Committee of the NHO Osaka Minami Medical Center in accordance with the Institutional Guide for the Care and Use of Laboratory Animals.
We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 131 kb)
Rights and permissions
About this article
Cite this article
Saeki, Y., Okita, Y., Igashira-Oguro, E. et al. Modulation of TNFR 1-triggered two opposing signals for inflammation and apoptosis via RIPK 1 disruption by geldanamycin in rheumatoid arthritis. Clin Rheumatol 40, 2395–2405 (2021). https://doi.org/10.1007/s10067-021-05579-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05579-w