Abstract
Objective
To evaluate the efficacy and safety of rituximab (RTX), an antiB cell monoclonal antibody, on lung and skin involvement in systemic sclerosis (SSc).
Methods
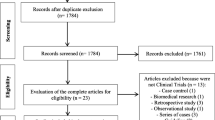
All literature published in Embase and Medline before September 2019 were comprehensively searched. Two independent reviewers selected eligible studies, extracted relevant data, and assessed the quality of the included studies. We only considered randomized, controlled trials (RCTs), cohort studies, and case-control studies that compared RTX with a placebo, other immunosuppressive agents, or corticosteroids. All analyses were performed using RevMan (version 5.3).
Results
A total of 8 studies (3 RCTs and 5 cohort studies) met our inclusion criteria. The pooled analysis showed a significant improvement of modified Rodnan skin score in the RTX group only in the cohort studies (mean difference [SD] − 3.31 [− 4.95, − 1.68]; I2 = 82%). As to the PFT, the RTX group showed a significant improvement in the forced vital capacity only in 3 RCTs (mean difference [SD] 6.59 [3.51, 9.68]; I2 = 0%). Additionally, the RTX group demonstrated a statistically significant improvement in the diffuse capacity of carbon monoxide only in the cohort studies (mean difference [SD] 7.42 [1.08, 13.76]; I2 = 97%). There were no significant differences in the AEs of the RTX and control groups.
Conclusions
RTX may be effective for lung and skin involvement in SSc, with no serious AEs. However, further studies with high quality and a large sample size are necessary to firmly establish the efficacy and safety of the use of RTX with SSc patients.
Key Points • RTX may be an alternative treatment for cutaneous and pulmonary manifestations in patients with SSc with a favorable safety profile. • However, further studies with a high quality and large sample size are necessary to firmly establish its efficacy and safety. |





Similar content being viewed by others
References
Rubio-Rivas M, Royo C, Simeon CP, Corbella X, Fonollosa V (2014) Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 44:208–219. https://doi.org/10.1016/j.semarthrit.2014.05.010
Denton CP, Black CM, Abraham DJ (2006) Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol 2:134–144. https://doi.org/10.1038/ncprheum0115
Katsumoto TR, Whitfield ML, Connolly MK (2011) The pathogenesis of systemic sclerosis. Annu Rev Pathol 6:509–537. https://doi.org/10.1146/annurev-pathol-011110-130312
Lafyatis R, O'Hara C, Feghali-Bostwick CA, Matteson E (2007) B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum 56 :3167-3168. https://doi.org/10.1002/art.22847
Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M et al (2006) B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol 169:954–966. https://doi.org/10.2353/ajpath.2006.060205
Kazkaz H, Isenberg D (2004) Anti B cell therapy (rituximab) in the treatment of autoimmune diseases. Curr Opin Pharmacol 4:398–402. https://doi.org/10.1016/j.coph.2004.03.006
Harrison AM, Thalji NM, Greenberg AJ, Tapia CJ, Windebank AJ (2014) Rituximab for non-Hodgkin’s lymphoma: a story of rapid success in translation. Clin Transl Sci 7:82–86. https://doi.org/10.1111/cts.12111
Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M et al (2017) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 76:960–977. https://doi.org/10.1136/annrheumdis-2016-210715
Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P et al (2014) Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371 :1771-1780. https://doi.org/10.1056/NEJMoa1404231
Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS et al (2010) Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363:221–232. https://doi.org/10.1056/NEJMoa0909905
Bosello S, De Santis M, Lama G, Spano C, Angelucci C, Tolusso B et al (2010) B cell depletion in diffuse progressive systemic sclerosis: safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open-label trial. Arthritis Res Ther 12:R54. https://doi.org/10.1186/ar2965
Bosello SL, De Luca G, Rucco M, Berardi G, Falcione M, Danza FM et al (2015) Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthritis Rheum 44:428–436. https://doi.org/10.1016/j.semarthrit.2014.09.002
Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Paliogianni F, Sirinian C et al (2012) Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol 30:S17–S22
Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T et al (2017) A multicenter, open-label, comparative study of B-cell depletion therapy with Rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum 46:625–631. https://doi.org/10.1016/j.semarthrit.2016.10.003
Lafyatis R, Kissin E, York M, Farina G, Viger K, Fritzler MJ et al (2009) B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum 60:578–583. https://doi.org/10.1002/art.24249
Melsens K, Vandecasteele E, Deschepper E, Badot V, Blockmans D, Brusselle G et al (2018) Two years follow-up of an open-label pilot study of treatment with rituximab in patients with early diffuse cutaneous systemic sclerosis. Acta Clin Belg 73:119–125. https://doi.org/10.1080/17843286.2017.1372244
Moazedi-Fuerst FC, Kielhauser SM, Brickmann K, Hermann J, Lutfi A, Meilinger M et al (2014) Rituximab for systemic sclerosis: arrest of pulmonary disease progression in five cases. Results of a lower dosage and shorter interval regimen. Scand J Rheumatol 43:257–258. https://doi.org/10.3109/03009742.2013.869617
Smith V, Piette Y, van Praet JT, Decuman S, Deschepper E, Elewaut D et al (2013) Two-year results of an open pilot study of a 2-treatment course with rituximab in patients with early systemic sclerosis with diffuse skin involvement. J Rheumatol 40:52–57. https://doi.org/10.3899/jrheum.120778
Smith V, Van Praet JT, Vandooren B, Van der Cruyssen B, Naeyaert JM, Decuman S et al (2010) Rituximab in diffuse cutaneous systemic sclerosis: an open-label clinical and histopathological study. Ann Rheum Dis 69:193–197. https://doi.org/10.1136/ard.2008.095463
Boonstra M, Meijs J, Dorjee AL, Marsan NA, Schouffoer A, Ninaber MK et al (2017) Rituximab in early systemic sclerosis. RMD Open 3:e000384
Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C et al (2010) Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 49:271–280. https://doi.org/10.1093/rheumatology/kep093
Phumethum V, Jamal S, Johnson SR (2011) Biologic therapy for systemic sclerosis: A systematic review. J Rheumatol 38:289–296. https://doi.org/10.3899/jrheum.100361
Young A, Khanna D (2015) Systemic sclerosis: a systematic review on therapeutic management from 2011 to 2014. Curr Opin Rheumatol 27:241–248. https://doi.org/10.1097/bor.0000000000000172
Masi AT, Diagnostic SFSCotARA, Committee TC (1980) Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23:581–590. https://doi.org/10.1002/art.1780230510
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A et al (2013) 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum 65 (:2737-2747. https://doi.org/10.1002/art.38098
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ d5928:343. https://doi.org/10.1136/bmj.d5928
Wells G, Shea B, O'Connell J (2014) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute Web site 7
Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P (2018) Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology 57:2106–2113
Ebata S, Yoshizaki A, Fukasawa T, Miura S, Takahashi T, Sumida H et al (2019) Rituximab therapy is more effective than cyclophosphamide therapy for Japanese patients with anti-topoisomerase I-positive systemic sclerosis-associated interstitial lung disease. J Dermatol 0. https://doi.org/10.1111/1346-8138.15079
Elhai M, Boubaya M, Distler O, Smith V, Matucci-Cerinic M, Alegre Sancho JJ et al (2019) Outcomes of patients with systemic sclerosis treated with rituximab in contemporary practice: a prospective cohort study. Ann Rheum Dis 78:979–987
Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y et al (2015) Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis 74:1188–1194
Thiebaut M, Launay D, Riviere S, Mahevas T, Bellakhal S, Hachulla E et al (2018) Efficacy and safety of rituximab in systemic sclerosis: French retrospective study and literature review. Autoimmun Rev 17:582–58733
Tang R, Yu J, Shi Y, Zou P, Zeng Z, Tang B et al (2020) Safety and efficacy of Rituximab in systemic sclerosis: a systematic review and meta-analysis. Int Immunopharmacol. 83:106389. https://doi.org/10.1016/j.intimp.2020.106389
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Sasimon Borrirukwisitsak performed the literature search. All authors performed study selection, data abstraction, and data analysis. The first draft of the manuscript was written by Sasimon Borrirukwisitsak and all authors commented on previous versions of the manuscript. Wanruchada Katchamart critically revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Ethics approval
This is a systematic review and metaanalysis that complies with a “Research with Exemption” category. The Siriraj Institutional Review Broad has confirmed that no ethical approval is required [Protocol number 210/2562 (Exempt), Date of Proof: April 2, 2019].
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code Availability
Review Manager software (version 5.3).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 901 kb)
Rights and permissions
About this article
Cite this article
Borrirukwisitsak, S., Tantayakom, P. & Katchamart, W. Efficacy and safety of rituximab on lung and skin involvement in systemic sclerosis: a systematic review and metaanalysis. Clin Rheumatol 40, 2779–2789 (2021). https://doi.org/10.1007/s10067-020-05542-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05542-1