Abstract
Introduction/objective
Thrombotic microangiopathy (TMA) in systemic lupus erythematosus is a rare manifestation associated with activation of the complement system. This study aimed to compare plasma and urine complement activation products between patients with active lupus nephritis (aLN) and those with acute TMA plus concomitant active LN (aTMA+aLN).
Methods
Plasma and urine samples were obtained from 20 patients with aTMA+aLN, 20 patients with aLN matched by the histological activity index, 5 patients with chronic TMA, 20 patients with inactive LN, and 10 kidney donors. Complement fragments C3a, C4a, C4d, Ba, C5a, C5bC9, and factor H were determined by ELISA; and kidney C4d deposition was detected by immunohistochemistry. Patients were followed for > 12 months and complement activation products re-measured after treatment in 10 aTMA+aLN patients.
Results
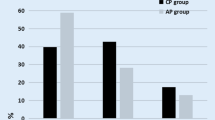
Both aTMA+aLN and aLN groups had increased circulating C3a, Ba, and C5bC9; and decreased circulating C3, C4, C4a, C4d, and factor H. Urinary C3a, C5a, Ba, and C5bC9 were higher in patients with aTMA+aLN than in aLN. After treatment, levels of circulating C3, C4, and factor H increased; while levels of urinary C3a, C5a, Ba, and C5bC9 decreased in patients with aTMA+aLN. These changes were observed at each aTMA episode in two patients studied during repeated TMA episodes. There was no difference in C4d deposition in glomerular capillaries, tubular basement membrane, peritubular capillaries, and arterioles between patients with aLN and those aTMA+aLN.
Conclusions
Circulating and urine complement activation products suggest that thrombotic microangiopathy associated with LN is mediated through activation of the alternative complement pathway.
Key Points • Immune-complex kidney disease in systemic lupus erythematosus (SLE) is associated with activation of the classical, lectin, and alternative complement pathways • Indirect evidence from measurement of circulating and urinary complement pathway activation products suggests that renal acute thrombotic microangiopathy in SLE is mediated by activation of the alternative complement pathway • C4d kidney immunohistochemistry may be positive in both immune complex nephritis and thrombotic microangiopathy. Therefore, it is not a specific marker of renal thrombotic microangiopathy in SLE. |



Similar content being viewed by others
References
Mejia-Vilet JM, Rovin BH (2019) Chapter 59: Epidemiology and management of lupus nephritis. In: Wallace D, Hahn B (ed) Dubois’ lupus erythematosus and related syndromes, 9th edn. Elsevier, pp 59_1–59_18
Wu LH, Yu F, Tan Y, Qu Z, Chen MH, Wang SX, Liu G, Zhao MH (2013) Inclusion of renal vascular lesions in the 2003 ISN/RPS system for classifying lupus nephritis improves renal outcome predictions. Kidney Int 83:715–723
Mejía-Vilet JM, Córdova-Sánchez BM, Uribe-Uribe NO, Correa-Rotter R, Morales-Buenrostro LE (2017) Prognostic significance of renal vascular pathology in lupus nephritis. Lupus 26:1042–1050
Brocklebank V, Wood KM, Kavanagh D (2018) Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol 13:300–317
Gavriilaki E, Anagnostopoulos A, Mastellos DC (2019) Complement in thrombotic microangiopathies: unraveling Ariadne’s thread into the labyrinth of complement therapeutics. Front Immunol 10:337
Ricklin D, Hajishengallis G, Yang K, Lambris JD (2010) Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797
Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Köhl J (2009) The role of the anaphylatoxins in health and disease. Mol Immunol 46:2753–2766
Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, Boackle SA, Colten HR, Gilkeson GS (2000) Modulation of renal disease in MRL/ lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol 164:786–794
Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS (2004) Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int 65:129–138
Bao L, Osawe I, Haas M, Quigg RJ (2005) Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol 175:1947–1955
Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ (2005) C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol 35:2496–2506
Belmont HM, Hopkins P, Edelson HS, Kaplan HB, Ludewig R, Weissmann G, Abramson S (1986) Complement activation during systemic lupus erythematosus. C3a and C5a anaphylatoxins circulate during exacerbations of disease. Arthritis Rheum 29:1085–1089
Buyon JP, Tamerius J, Belmont HM, Abramson SB (1992) Assessment of disease activity and impending flare in patients with systemic lupus erythematosus. Comparison of the use of complement split products and conventional measurements of complement. Arthritis Rheum 35:1028–1037
Manzi S, Rairie JE, Carpenter AB, Kelly RH, Jagarlapudi SP, Sereika SM, Medsger TA Jr, Ramsey-Goldman R (1996) Sensitivity and specificity of plasma and urine complement split products as indicators of lupus disease activity. Arthritis Rheum 39:1178–1188
Song D, Guo WY, Wang FM, Li YZ, Song Y, Yu F, Zhao MH (2017) Complement alternative pathway’s activation in patients with lupus nephritis. Am J Med Sci 353:247–257
Martin M, Smolag KI, Björk A, Gullstrand B, Okrój M, Leffler J, Jönsen A, Bengtsson AA, Blom AM (2017) Plasma C4d as marker for lupus nephritis in systemic lupus erythematosus. Arthritis Res Ther 19:266
Nisihara RM, Magrini F, Mocelin V, Messias-Reason IJ (2013) Deposition of the lectin pathway of complement in renal biopsies of lupus nephritis patients. Hum Immunol 74:907–910
Kim SH, Jeong HJ (2003) Glomerular C4d deposition indicates in situ classic complement pathway activation, but is not a marker for lupus nephritis activity. Yonsei Med J 44:75–80
Li SJ, Liu ZH, Zen CH, Wang QW, Wang Y, Li LS (2007) Peritubular capillary C4d deposition in lupus nephritis different from antibody-mediated renal rejection. Lupus 16:875–880
Sato N, Ohsawa I, Nagamachi S, Ishii M, Kusaba G, Inoshita H, Toki A, Horikoshi S, Ohi H, Matsushita M, Tomino Y (2011) Significance of glomerular activation of the alternative pathway and lectin pathway in lupus nephritis. Lupus 20:1378–1386
Batal I, Liang K, Bastacky S, Kiss LP, McHale T, Wilson NL, Paul B, Lertratanakul A, Ahearn JM, Manzi S, Kao AH (2012) Prospective assessment of C4d deposits on circulating cells and renal tissues in lupus nephritis: a pilot study. Lupus 21:13–26
Sahin OZ, Gurses S, Tasli F, Yavas H, Ersoy R, Uzum A, Cirit M (2013) Glomerular C4d staining can be an indicator of disease activity in lupus nephritis. Ren Fail 35:222–225
Cohen D, Koopmans M, Kremer Hovinga IC, Berger SP, van Groningen MR, Steup-Beekman GM, de Heer E, Bruijn JA, Bajema IM (2008) Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum 58:2460–2469
Shen Y, Chen XW, Sun CY, Dai M, Yan YC, Yang CD (2010) Association between anti-β2 glycoprotein I antibodies and renal glomerular C4d deposition in lupus nephritis patients with glomerular microthrombosis: a prospective study of 155 cases. Lupus 19:1195–1203
Chua JS, Baelde HJ, Zandbergen M, Wilhelmus S, van Es LA, de Fijter JW, Bruijn JA, BAjema IM, Cohen D (2015) Complement factor C4d is a common denominator in thrombotic microangiopathy. J Am Soc Nephrol 26:2239–2247
Appel GB, Pirani CL, D’Agati V (1994) Renal vascular complications of systemic. J Am Soc Nephrol 4:1499–1515
Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D’Agati VD, Ferrario F, Haas M, Jennette JC, Joh K, Nast CC, Noël LH, Rijnink EC, Roberts ISD, Seshan SV, Sethi S, Fogo AB (2018) Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 93:789–796
Birmingham DJ, Hebert LA (2015) The complement system in lupus nephritis. Semin Nephrol 35:444–454
Manderson AP, Botto M, Walport MJ (2004) The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol 22:431–456
Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC (2005) C5a receptor deficiency attenuates T cell function and renal disease in MRL lpr mice. J Am Soc Nephrol 16:3572–3582
Wenderfer SE, Wang H, Ke B, Wetsel RA, Braun MC (2009) C3a receptor deficiency accelerates the onset of renal injury in the MRL/lpr mouse. Mol Immunol 46:1397–1404
Kusunoki Y, Akutsu Y, Itami N, Tochimaru H, Nagata Y, Takekoshi Y, Sagawa A, Kataoka Y, Nagasawa S (1991) Urinary excretion of terminal complement complexes in glomerular disease. Nephron 59:27–32
Gou SJ, Yuan J, Wang C, Zhao MH, Chen M (2013) Alternative Complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol 8:1884–1891
de Jorge EG, Macor P, Paixão-Cavalcante D, Rose KL, Tedesco F, Cook HT, Botto M, Pickering M (2011) The development of atypical hemolytic uremic syndrome depends on complement C5. J Am Soc Nephrol 22:137–145
Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G (2007) Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody–induced fetal injury. Blood 110:2423–2431
Saadi S, Holzknecht RA, Patte CP, Stern DM, Platt JL (1995) Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med 182:1807–1814
Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J (2005) Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum 52:2120–2124
Oku K, Atsumi T, Bohgaki M, Amengual O, Kataoka H, Horita T, Yasuda S, Koike T (2009) Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis 68:1030–1035
Breen KA, Seed P, Parmar K, Moore GW, Stuart-Smith SE, Hunt BJ (2012) Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thromb Haemost 107:423–429
Rand JH, Wu XX, Wolgast LR, Lei V, Conway EM (2017) A novel 2-stage approach that detects complement activation in patients with antiphospholipid antibody syndrome. Thromb Res 156:119–125
Kello N, El Khoury L, Marder G, Furie R, Zapantis E, Horowitz DL (2019) Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: Case series and review of literature. Semin Arthritis Rheum 49:74–83
Bao L, Haas M, Quigg RJ (2011) Complement factor H deficiency accelerates development of lupus nephritis. J Am Soc Nephrol 22:285–295
Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, Kaufman KM, Langefeld CD, Williams AH, Comeau ME, Ziegler JT, Marion MC, Adler A, Glenn SB, Alarcón-Riquelme ME, BIOLUPUS Network, GENLES Network, Pons-Estel BA, Harley JB, Bae SC, Bang SY, Cho SK, Jacob CO, Vyse TJ, Niewold TB, Gaffney PM, Moser KL, Kimberly RP, Edberg JC, Brown EE, Alarcon GS, Petri MA, Ramsey-Goldman R, Vilá LM, Reveille JD, James JA, Gilkeson GS, Kamen DL, Freedman BI, Anaya JM, Merrill JT, Criswell LA, Scofield RH, Stevens AM, Guthridge JM, Chang DM, Song YW, Park JA, Lee EY, Boackle SA, Grossman JM, Hahn BH, Goodship THJ, Cantor RM, Yu CY, Shen N, Tsao BP (2011) Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet 7:e1002079
Wang F, Yu F, Tan Y, Song D, Zhao M (2012) Serum complement factor H is associated with clinical and pathological activities of patients with lupus nephritis. Rheumatology (Oxford) 51:2269–2277
Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM (2012) Pros and cons for C4d as a biomarker. Kidney Int 81:628–639
Acknowledgments
This study was performed in partial fulfillment for the Doctorate Program in Medical Sciences of the Universidad Nacional Autónoma de México (UNAM). Juan M. Mejia-Vilet is a doctoral student from this program.
Funding
This work was supported by the Mexican National Council of Science and Technology (CONACYT), FOSSIS 2017-2-289663 grant to Juan M. Mejia-Vilet
Author information
Authors and Affiliations
Contributions
Conception and study design: JMMV, IGR, LEMB. Patient recruitment, sample and data acquisition, and management: JMMV, IGR, CC, RAMP, RACB, NOUU, CNA. Laboratory analyses: CC, NOUU, CNA. Analysis and interpretation of data: JMMV, IGR, LEMB. Drafting and revising of the manuscript: all authors.
Corresponding author
Ethics declarations
This research was conducted in compliance with the Helsinki declaration. The study was approved by the local research and ethics boards.
Disclosures
None.
Ethical standard statement
The manuscript does not contain clinical studies or patient data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 18563 kb)
Rights and permissions
About this article
Cite this article
Mejia-Vilet, J.M., Gómez-Ruiz, I.A., Cruz, C. et al. Alternative complement pathway activation in thrombotic microangiopathy associated with lupus nephritis. Clin Rheumatol 40, 2233–2242 (2021). https://doi.org/10.1007/s10067-020-05499-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05499-1