Abstract
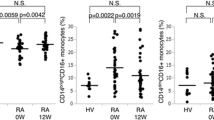
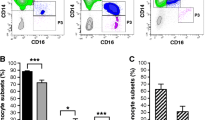
As the precursors of macrophages and osteoclasts, monocytes play an important role in the pathogenesis of rheumatoid arthritis (RA). Since the deficiency of zinc-finger protein A20 in myeloid cells triggers erosive polyarthritis resembling RA, A20 in monocytes may play a protective role in RA. In the present study, we aimed to investigate the abnormality of monocyte subtypes and the expression of zinc-finger protein A20 in RA. Peripheral blood mononuclear cells and clinical data were collected from RA patients and healthy controls (HCs). Monocyte subtypes and A20 expression were determined through flow cytometry and compared between the two groups. Correlations between monocyte subtypes, A20 expression, and clinical data were analyzed. A total of 43 RA patients and 23 HCs were included in the present study. RA patients had higher absolute monocyte counts (p < 0.001) in the peripheral blood. The proportions and counts of intermediate monocytes (IMs) (both p < 0.001) and non-classical monocytes (NCMs) were higher (both p < 0.001) in RA patients. The expression of A20 in IMs (p < 0.001) was lower in RA patients compared with that in the HCs. Furthermore, the expression of A20 in IMs was negatively correlated with the anti-cyclic citrullinated peptide (CCP) antibody level in RA patients (r = − 0.409, p = 0.01). The expression of A20 in NCMs was positively correlated with modified total Sharp score (mTSS) in RA patients (r = 0.471, p = 0.02). Collectively, we proved that IMs and NCMs were increased in RA patients, suggesting that they played a suggestive role in the pathogenesis of RA. Furthermore, the downregulation of A20 in IMs might be correlated with anti-CCP antibody production. The A20 expression in NCMs might affect bone erosion in RA.
Key Points • IMs and NCMs were increased in the peripheral blood of RA patients, suggesting their pathogenic role in RA. • The decreased expression of zinc-finger protein A20 in IMs of RA patients suggested the protective role of A20 in RA. • The negative correlation between the A20 expression in IMs and anti-CCP antibody revealed that A20 in IMs might be related to the formation of anti-CCP antibodies. • The positive correlation between the A20 expression in NCMs and mTSS revealed that A20 in NCMs might affect the bone erosion in RA. |






Similar content being viewed by others
References
Ciccacci C, Latini A, Perricone C, Conigliaro P, Colafrancesco S, Ceccarelli F, Priori R, Conti F, Perricone R, Novelli G, Borgiani P (2019) TNFAIP3 gene polymorphisms in three common autoimmune diseases: systemic lupus erythematosus, rheumatoid arthritis, and primary Sjogren syndrome-association with disease susceptibility and clinical phenotypes in Italian patients. J Immunol Res 2019:6728694–6728696. https://doi.org/10.1155/2019/6728694
López-Zambrano M, Rodriguez-Montesinos J, Crespo-Avilan GE, Muñoz-Vega M, Preissner KT (2020) Thrombin promotes macrophage polarization into M1-like phenotype to induce inflammatory responses. Thromb Haemost 120:658–670. https://doi.org/10.1055/s-0040-1703007
Zhao C, Zhang L, Kong W, Liang J, Xu X, Wu H, Feng X, Hua B, Wang H, Sun L (2015) Umbilical cord-derived mesenchymal stem cells inhibit cadherin-11 expression by fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol Res 2015:137695–137610. https://doi.org/10.1155/2015/137695
Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL (2019) Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol 10:2035. https://doi.org/10.3389/fimmu.2019.02035
Lacerte P, Brunet A, Egarnes B, Duchêne B, Brown JP, Gosselin J (2016) Overexpression of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients contributes to enhance responsiveness to TLR agonists. Arthritis Res Ther 18:10. https://doi.org/10.1186/s13075-015-0901-1
Laurent L, Clavel C, Lemaire O, Anquetil F, Cornillet M, Zabraniecki L, Nogueira L, Fournié B, Serre G, Sebbag M (2011) Fcγ receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann Rheum Dis 70(6):1052–1059. https://doi.org/10.1136/ard.2010.142091
Klimek E, Mikołajczyk T, Sulicka J, Kwaśny-Krochin B, Korkosz M, Osmenda G, Wizner B, Surdacki A, Guzik T, Grodzicki TK, Skalska A (2014) Blood monocyte subsets and selected cardiovascular risk markers in rheumatoid arthritis of short duration in relation to disease activity. Biomed Res Int 2014:736853–736810. https://doi.org/10.1155/2014/736853
Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Guire CM, Vereecke L, Chu Y, Boon L, Staelens S, Matthys P, Lambrecht BN, Schmidt-Supprian M, Pasparakis M, Elewaut D, Beyaert R, van Loo G (2011) A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet 43(9):908–912. https://doi.org/10.1038/ng.874
Majumdar I, Paul J (2014) The deubiquitinase A20 in immunopathology of autoimmune diseases. Autoimmunity 47(5):307–319. https://doi.org/10.3109/08916934.2014.900756
Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A (2000) Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289(5488):2350–2354. https://doi.org/10.1126/science.289.5488.2350
Vande Walle L, van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti TD, van Loo G, Lamkanfi M (2014) Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512(7512):69–73. https://doi.org/10.1038/nature13322
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69(9):1580–1588. https://doi.org/10.1136/ard.2010.138461
Rana AK, Li Y, Dang Q, Yang F (2018) Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int Immunopharmacol 65:348–359. https://doi.org/10.1016/j.intimp.2018.10.016
Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM (2017) Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One 12(4):e0176460. https://doi.org/10.1371/journal.pone.0176460
de La Rica L, García-Gómez A, Comet NR et al (2015) NF-κB-direct activation of microRNAs with repressive effects on monocyte-specific genes is critical for osteoclast differentiation. Genome Biol 16:2. https://doi.org/10.1186/s13059-014-0561-5
Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U (2012) The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 64(3):671–677. https://doi.org/10.1002/art.33418
Tsukamoto M, Seta N, Yoshimoto K, Suzuki K, Yamaoka K, Takeuchi T (2017) CD14brightCD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res Ther 19(1):28. https://doi.org/10.1186/s13075-016-1216-6
Yoon BR, Yoo S-J, Choi Y et al (2014) Functional phenotype of synovial monocytes modulating inflammatory T-cell responses in rheumatoid arthritis (RA). PLoS One 9(10):e109775. https://doi.org/10.1371/journal.pone.0109775
Winchester R, Giles JT, Nativ S et al (2016) Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol 68(1):92–102. https://doi.org/10.1002/art.39419
Ong S-M, Hadadi E, Dang T-M, Yeap WH, Tan CTY, Ng TP, Larbi A, Wong SC (2018) The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis 9(3):266. https://doi.org/10.1038/s41419-018-0327-1
Tu J, Hong W, Zhang P, Wang X, Körner H, Wei W (2018) Ontology and function of fibroblast-like and macrophage-like synoviocytes: how do they talk to each other and can they be targeted for rheumatoid arthritis therapy? Front Immunol 9:1467. https://doi.org/10.3389/fimmu.2018.01467
Moaaz M, Mohannad N (2016) Association of the polymorphisms of TRAF1 (rs10818488) and TNFAIP3 (rs2230926) with rheumatoid arthritis and systemic lupus erythematosus and their relationship to disease activity among Egyptian patients. Cent Eur J Immunol 41(2):165–175. https://doi.org/10.5114/ceji.2016.60991
Dong X, Zheng Z, Zhai Y, Zheng Y, Ding J, Jiang J, Zhu P (2018) ACPA mediates the interplay between innate and adaptive immunity in rheumatoid arthritis. Autoimmun Rev 17(9):845–853. https://doi.org/10.1016/j.autrev.2018.02.014
Yamada R (2005) Peptidylarginine deiminase type 4, anticitrullinated peptide antibodies, and rheumatoid arthritis. Autoimmun Rev 4(4):201–206. https://doi.org/10.1016/j.autrev.2004.11.002
Odqvist L, Jevnikar Z, Riise R, Öberg L, Rhedin M, Leonard D, Yrlid L, Jackson S, Mattsson J, Nanda S, Cohen P, Knebel A, Arthur S, Thörn K, Svenungsson E, Jönsen A, Gunnarsson I, Tandre K, Alexsson A, Kastbom A, Rantapää-Dahlqvist S, Eloranta ML, Syvänen AC, Bengtsson A, Johansson P, Sandling JK, Sjöwall C, Rönnblom L, Collins B, Vaarala O (2019) Genetic variations in A20 DUB domain provide a genetic link to citrullination and neutrophil extracellular traps in systemic lupus erythematosus. Ann Rheum Dis 78(10):1363–1370. https://doi.org/10.1136/annrheumdis-2019-215434
Cooper DL, Martin SG, Robinson JI, Mackie SL, Charles CJ, Nam J, Consortium YEAR, Isaacs JD, Emery P, Morgan AW (2012) FcγRIIIa expression on monocytes in rheumatoid arthritis: role in immune-complex stimulated TNF production and non-response to methotrexate therapy. PLoS One 7(1):e28918. https://doi.org/10.1371/journal.pone.0028918
Puchner A, Saferding V, Bonelli M, Mikami Y, Hofmann M, Brunner JS, Caldera M, Goncalves-Alves E, Binder NB, Fischer A, Simader E, Steiner CW, Leiss H, Hayer S, Niederreiter B, Karonitsch T, Koenders MI, Podesser BK, O’Shea JJ, Menche J, Smolen JS, Redlich K, Blüml S (2018) Non-classical monocytes as mediators of tissue destruction in arthritis. Ann Rheum Dis 77(10):1490–1497. https://doi.org/10.1136/annrheumdis-2018-213250
Lee MJ, Lim E, Mun S et al (2016) Intravenous immunoglobulin (IVIG) attenuates TNF-induced pathologic bone resorption and suppresses osteoclastogenesis by inducing A20 expression. J Cell Physiol 231(2):449–458. https://doi.org/10.1002/jcp.25091
Mabilleau G, Chappard D, Sabokbar A (2011) Role of the A20-TRAF6 axis in lipopolysaccharide-mediated osteoclastogenesis. J Biol Chem 286(5):3242–3249. https://doi.org/10.1074/jbc.M110.150300
Acknowledgments
We thank all the participants and volunteers who have so willingly participated in this study, thus making this study possible.
Funding
This study was financially supported by the National Natural Science Foundation of China (81302585), Scientific Research Projects for Top-notch Talents in Jiangsu Province (LGY2019023), the Jiangsu Postdoctoral Research Support Program (2019K260), Scientific Research Project of Maternal and Child Health in Jiangsu Province (F201837), Science and Technology Project of Changzhou Health Committee for Young Talents (QN201910, QN201805), the Key Programs of Changzhou City Commission for Discipline Inspection (ZD201707, ZD201607, ZD201108), and the Research Project of Jiangsu Modern Hospital Management (JSY-3-2019-107).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University, and all participants gave their consents.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Study flow diagram (PDF 70 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Yao, Y., Tian, J. et al. Alterations and abnormal expression of A20 in peripheral monocyte subtypes in patients with rheumatoid arthritis. Clin Rheumatol 40, 341–348 (2021). https://doi.org/10.1007/s10067-020-05137-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05137-w