Abstract
Background
Tumor necrosis factor-α (TNFα) inhibitors (TNFi) have greatly improved the prognosis of RA and become the first therapeutic option for patients who failed the conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) therapy, but not all these patients respond well to TNFi. So far, there has been no definite biomarker to predict the response to TNFi yet.
Methods
Sixty rheumatoid arthritis (RA) patients with disease duration more than 6 months and at least low disease activity defined by DAS28-CRP > 3.2 although after csDMARDs (including MTX and/or leflunomide) treatment for more than 3 months were included. They were further treated with TNFα receptor Fc fusion protein and MTX 10 mg per week for 12 weeks. Soluble ICAM-1 (sICAM-1) and CXCL13 concentrations in sera from 60 RA patients and 20 healthy controls were tested by ELISA right before and at the end of 12 weeks of TNFi therapy. The correlation between sICAM-1 and CXCL13 with disease activity and their predictive values for TNFi response were analyzed.
Results
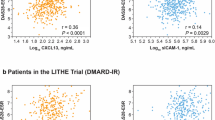
The mean age of the 60 patients was 54.8 ± 11.6 years. Serum sICAM-1 and CXCL13 concentrations were higher in RA patients than heathy controls, higher in seropositive RA patients than in seronegative ones, and higher in RA patients with higher disease activity. Serum sICAM-1 and CXCL13 levels were decreased after TNFi therapy, especially in good responders. Baseline sICAM-1 concentration was independently associated with the EULAR response (p = 0.033, OR = 1.014, 95% CI = 1.003–1.026). The sICAM-1high/CXCL13high patients had the highest response rate, which was significantly higher than the sICAM-1low/CXCL13low group (OR = 8.143, 95% CI = 1.040–75.482, p = 0.045).
Conclusion
sICAM-1 and CXCL13 are elevated in RA patients and correlated with disease activity. sICAM-1 is an independent predictor of TNFi response in csDMARDs refractory RA patients.
Key Points • This study confirmed the predictive value of soluble ICAM-1 (sICAM-1) and CXCL13 on the response to TNFi in RA patient. • Baseline sICAM-1 concentration was independently associated with the EULAR response. • The sICAM-1high/CXCL13high patients had significantly higher response rate than the sICAM-1low/CXCL13low group. |




Similar content being viewed by others
References
Katchamart W, Trudeau J, Phumethum V, Bombardier C (2009) Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 68:1105–1112
Scott DL, Kingsley GH (2006) Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med 355:704–712
Braun-Moscovici Y, Markovits D, Zinder O, Schapira D, Rozin A, Ehrenburg M, Dain L, Hoffer E, Nahir AM, Balbir-Gurman A (2006) Anti-cyclic citrullinated protein antibodies as a predictor of response to anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis. J Rheumatol 33:497–500
Canet LM, Sánchez-Maldonado JM, Cáliz R, Rodríguez-Ramos A, Lupiañez CB, Canhão H, Martínez-Bueno M, Escudero A, Segura-Catena J, Sorensen SB, Hetland ML, Soto-Pino MJ, Ferrer MA, García A, Glintborg B, Filipescu I, Pérez-Pampin E, González-Utrilla A, Nevot MÁL, Conesa-Zamora P, Broeder AD, de Vita S, Jacobsen SEH, Collantes-Estevez E, Quartuccio L, Canzian F, Fonseca JE, Coenen MJH, Andersen V, Sainz J (2019) Polymorphisms at phase I-metabolizing enzyme and hormone receptor loci influence the response to anti-TNF therapy in rheumatoid arthritis patients. Pharm J 19:83–96
Jenkins JK, Hardy KJ, McMurray RW (2002) The pathogenesis of rheumatoid arthritis: a guide to therapy. Am J Med Sci 323:171–180
Odai T, Matsunawa M, Takahashi R, Wakabayashi K, Isozaki T, Yajima N, Miwa Y, Kasama T (2009) Correlation of CX3CL1 and CX3CR1 levels with response to infliximab therapy in patients with rheumatoid arthritis. J Rheumatol 36:1158–1165
Dennis G Jr, Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA et al (2014) Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 16:R90
Han BK, Kuzin I, Gaughan JP, Olsen NJ, Bottaro A (2016) Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: a pilot, prospective study. Arthritis Res Ther 18:93
Greisen SR, Schelde KK, Rasmussen TK, Kragstrup TW, Stengaard-Pedersen K, Hetland ML et al (2014) CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic ‘window of opportunity’. Arthritis Res Ther 16:434
Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M et al (2015) CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol 16:6
Mandik-Nayak L, Huang G, Sheehan KC, Erikson J, Chaplin DD (2001) Signaling through TNF receptor p55 in TNF-alpha-deficient mice alters the CXCL13/CCL19/CCL21 ratio in the spleen and induces maturation and migration of anergic B cells into the B cell follicle. J Immunol 167:1920–1928
Kobayashi S, Murata K, Shibuya H, Morita M, Ishikawa M, Furu M, Ito H, Ito J, Matsuda S, Watanabe T, Yoshitomi H (2013) A distinct human CD4+ T cell subset that secretes CXCL13 in rheumatoid synovium. Arthritis Rheum 65:3063–3072
Jones JD, Hamilton BJ, Challener GJ, de Brum-Fernandes AJ, Cossette P, Liang P et al (2014) Serum C-X-C motif chemokine 13 is elevated in early and established rheumatoid arthritis and correlates with rheumatoid factor levels. Arthritis Res Ther 16:R103
Bugatti S, Manzo A, Benaglio F, Klersy C, Vitolo B, Todoerti M et al (2012) Serum levels of CXCL13 are associated with ultrasonographic synovitis and predict power Doppler persistence in early rheumatoid arthritis treated with non-biological disease-modifying anti-rheumatic drugs. Arthritis Res Ther 14:R34
Mcinnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219
Pino M, Galleguillos C, Torres M, Sovino H, Fuentes A, Boric MA, Johnson MC (2009) Association between MMP1 and MMP9 activities and ICAM1 cleavage induced by tumor necrosis factor in stromal cell cultures from eutopic endometria of women with endometriosis. Reproduction 138:837–847
Corsiero E, Bombardieri M, Manzo A, Bugatti S, Uguccioni M, Pitzalis C (2012) Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol Lett 145:62–67
Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M, Wilson AG, Binks MH, Dickson MC (2008) Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor alpha, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor. Arthritis Rheum 58:2257–2267
Ugur M, Yildirim K, Kiziltunc A, Erdal A, Karatay S, Senel K (2004) Correlation between soluble intercellular adhesion molecule 1 level and extracellular superoxide dismutase activity in rheumatoid arthritis: a possible association with disease activity. Scand J Rheumatol 33:239–243
Pandya JM, Lundell AC, Andersson K, Nordström I, Theander E, Rudin A (2017) Blood chemokine profile in untreated early rheumatoid arthritis: CXCL10 as a disease activity marker. Arthritis Res Ther 19:20
Mason JC, Kapahi P, Haskard DO (1993) Detection of increased levels of circulating intercellular adhesion molecule 1 in some patients with rheumatoid arthritis but not in patients with systemic lupus erythematosus. Lack of correlation with levels of circulating vascular cell adhesion molecule 1. Arthritis Rheum 36:519–527
Jonsson T, Arinbjarnarson S, Thorsteinsson J, Steinsson K, Geirsson AJ, Jónsson H et al (1995) Raised IgA rheumatoid factor (RF) but not IgM RF or IgG RF is associated with extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol 24:372–375
Ronnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L et al (2005) Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 64:1744–1749
Meeuwisse CM, Van Der Linden MP, Rullmann TA, Allaart CF, Nelissen R, Huizinga TW et al (2011) Identification of CXCL13 as a marker for rheumatoid arthritis outcome using an in silico model of the rheumatic joint. Arthritis Rheum 63:1265–1273
Den Broeder AA, Joosten A, Saxne T, Heinegård D, Fenner H, Miltenburg AM et al (2002) Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann Rheum Dis 61:311–318
Funding
This work was supported by the Peking University Clinical Research Program (PUCRP201305) and the National Natural Science Foundation of China (Nos. 81771740 and 81971524).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval was granted for this study, and consent was obtained from all the subjects. All patients provided their informed consent, and the study was conducted according to the principles of the Declaration of Helsinki.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, J., Ye, X. & Zhang, Z. The predictive value of serum soluble ICAM-1 and CXCL13 in the therapeutic response to TNF inhibitor in rheumatoid arthritis patients who are refractory to csDMARDs. Clin Rheumatol 39, 2573–2581 (2020). https://doi.org/10.1007/s10067-020-05043-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05043-1