Abstract
Introduction
Detection of autoantibodies in sera of rheumatoid arthritis (RA) patients has an important role in diagnosis and management strategies. Recently, another type of autoantibodies has been detected with activity against carbamylated proteins (anti-CarP) which may play an important role in the diagnosis of RA. The aim of this study was to raise knowledge about the diagnostic and prognostic value of anti-CarP antibodies in RA.
Materials and methods
Seventy RA patients and thirty-four controls were included in this study. DAS28 was used to evaluate disease activity. Joint erosions were assessed by Larsen score using plain X-ray of involved joints of hands and feet. Serum samples were analyzed for anti-CarP antibody titer using the ELISA technique.
Results
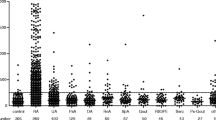
Out of 70 patients, 35.7% were positive for anti-CarP and only 5.88% of controls had high titer above the cut-off value. A total of 24.29% of the patients were RF-negative and 30% were ACPA-negative. Five patients (29.41%) of the negative RF group were positive for anti-CarP. Four patients (19%) of the ACPA-negative group were positive for anti-CarP, and three patients (4.28%) of the total number of patients were triple negative and seventeen (24.28%) were triple positive. There was a significant correlation between anti-CarP titer and both DAS28 and Larsen scores only in the positive anti-CarP group. In addition, there was a strong association between anti-CarP antibody titer and joint erosions at both baseline and after 1-year follow-up.
Conclusion
Presence of the anti-CarP antibodies in sera of RA patients may have a prognostic value as it correlates with the disease activity and joint erosions; moreover, it may have a diagnostic value in rheumatoid arthritis especially in RF- and ACPA-negative patients.
Key Points • This study was carried out to raise our knowledge about the importance of anti-CarP antibodies in predicting the prognosis of RA. • This study was carried out to assess the correlation between anti-CarP antibodies, disease activity, and joint erosions. • This study was carried out to state the extent to which we can rely on the anti-CarP antibodies as a biomarker for prediction of RA. |



Similar content being viewed by others
References
Derksen VF, Ajeganova S, Trouw LA, van der Helm-van Mil AH, Hafstrom I, Huizinga TW, Toes RE, Svensson B, van der Woude D (2017) Rheumatoid arthritis phenotype at presentation differs depending on the number of autoantibodies present. Ann Rheum Dis 76(4):716–720. https://doi.org/10.1136/annrheumdis-2016-209794
Lagrutta M, Alle G, Parodi RL, Greca AA (2016) Severe extra-articular manifestations of rheumatoid arthritis in absence of concomitant joint involvement following long-term spontaneous remission. A case report. Reumatol Clin 12(4):223–225. https://doi.org/10.1016/j.reuma.2015.07.006
Krougly LB, Fomicheva OA, Karpov YA, Popkova TV, Novikova DS, Nasonov EL (2016) Cardiovascular complications of rheumatoid arthritis: prevalence and pathogenesis. Kardiologiia 56(6):89–95
Onuora S (2012) Rheumatoid arthritis: RF levels predict RA risk in the general population. Nat Rev Rheumatol 8(10):562. https://doi.org/10.1038/nrrheum.2012.159
Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM (2017) Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol 31(1):3–18. https://doi.org/10.1016/j.berh.2017.08.003
Hensvold AH, Frisell T, Magnusson PK, Holmdahl R, Askling J, Catrina AI (2017) How well do ACPA discriminate and predict RA in the general population: a study based on 12 590 population-representative Swedish twins. Ann Rheum Dis 76(1):119–125. https://doi.org/10.1136/annrheumdis-2015-208980
Gafvels C, Hagerstrom M, Rane K, Wajngot A, Wandell PE (2016) Depression and anxiety after 2 years of follow-up in patients diagnosed with diabetes or rheumatoid arthritis. Health Psychol Open 3(2):2055102916678107. https://doi.org/10.1177/2055102916678107
Goes ACJ, Reis LAB, Silva MBG, Kahlow BS, Skare TL (2017) Rheumatoid arthritis and sleep quality. Rev Bras Reumatol 57(4):294–298. https://doi.org/10.1016/j.rbre.2016.07.011
Burmester GR, Pope JE (2017) Novel treatment strategies in rheumatoid arthritis. Lancet 389(10086):2338–2348. https://doi.org/10.1016/S0140-6736(17)31491-5
Oliveira RA, Fierro IM (2018) New strategies for patenting biological medicines used in rheumatoid arthritis treatment. Expert Opin Ther Pat 28(8):635–646. https://doi.org/10.1080/13543776.2018.1502748
Szodoray P, Szabo Z, Kapitany A, Gyetvai A, Lakos G, Szanto S, Szucs G, Szekanecz Z (2010) Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun Rev 9(3):140–143. https://doi.org/10.1016/j.autrev.2009.04.006
Vos I, Van Mol C, Trouw LA, Mahler M, Bakker JA, Van Offel J, De Clerck L, Huizinga TW (2017) Anti-citrullinated protein antibodies in the diagnosis of rheumatoid arthritis (RA): diagnostic performance of automated anti-CCP-2 and anti-CCP-3 antibodies assays. Clin Rheumatol 36(7):1487–1492. https://doi.org/10.1007/s10067-017-3684-8
Hoffman IE, Peene I, Cebecauer L, Isenberg D, Huizinga TW, Union A, Meheus L, De Bosschere K, Hulstaert F, Veys EM, De Keyser F (2005) Presence of rheumatoid factor and antibodies to citrullinated peptides in systemic lupus erythematosus. Ann Rheum Dis 64(2):330–332. https://doi.org/10.1136/ard.2004.022111
Vencovsky J (1991) Rheumatoid factor in rheumatoid arthritis in old age. Cas Lek Cesk 130(4):108–111
Bharadwaj A, Aggarwal A, Misra R (1999) Clinical relevance of IgA rheumatoid factor (RF) in children with juvenile rheumatoid arthritis. Rheumatol Int 19(1–2):47–49
Chatpar PC, Muller D, Gruber B, Kaplan AP, Gorevic PD (1986) Prevalence of IgE rheumatoid factor (IgE RF) in mixed cryoglobulinemia and rheumatoid arthritis. Clin Exp Rheumatol 4(4):313–317
Gioud-Paquet M, Auvinet M, Raffin T, Girard P, Bouvier M, Lejeune E, Monier JC (1987) IgM rheumatoid factor (RF), IgA RF, IgE RF, and IgG RF detected by ELISA in rheumatoid arthritis. Ann Rheum Dis 46(1):65–71
Pope JE, Movahedi M, Rampakakis E, Cesta A, Sampalis JS, Keystone E, Thorne C, Bombardier C (2018) ACPA and RF as predictors of sustained clinical remission in patients with rheumatoid arthritis: data from the Ontario Best practices Research Initiative (OBRI). RMD Open 4(2):e000738. https://doi.org/10.1136/rmdopen-2018-000738
Porto LS, Tavares WCJ, Costa DA, Lanna CC, Kakehasi AM (2017) Anti-CCP antibodies are not a marker of severity in established rheumatoid arthritis: a magnetic resonance imaging study. Rev Bras Reumatol 57(1):15–22. https://doi.org/10.1016/j.rbre.2015.07.018
Burgers LE, van Steenbergen HW, Ten Brinck RM, Huizinga TW, van der Helm-van Mil AH (2017) Differences in the symptomatic phase preceding ACPA-positive and ACPA-negative RA: a longitudinal study in arthralgia during progression to clinical arthritis. Ann Rheum Dis 76(10):1751–1754. https://doi.org/10.1136/annrheumdis-2017-211325
Hensvold AH, Catrina AI (2018) Challenging judgement of a low-positive ACPA test in the context of individuals at risk of RA. Ann Rheum Dis 77(6):e30. https://doi.org/10.1136/annrheumdis-2016-211062
Kalim S, Karumanchi SA, Thadhani RI, Berg AH (2014) Protein carbamylation in kidney disease: pathogenesis and clinical implications. Am J Kidney Dis 64(5):793–803. https://doi.org/10.1053/j.ajkd.2014.04.034
Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, Levarht NE, van der Helm-van Mil AH, Cerami A, Huizinga TW, Toes RE, Trouw LA (2011) Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A 108(42):17372–17377. https://doi.org/10.1073/pnas.1114465108
Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Hamann D, van Schaardenburg D, Toes RE, Trouw LA (2014) Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis 73(4):780–783. https://doi.org/10.1136/annrheumdis-2013-204154
Yee A, Webb T, Seaman A, Infantino M, Meacci F, Manfredi M, Benucci M, Lakos G, Favalli E, Schioppo T, Meroni PL, Mahler M (2015) Anti-CarP antibodies as promising marker to measure joint damage and disease activity in patients with rheumatoid arthritis. Immunol Res 61(1–2):24–30. https://doi.org/10.1007/s12026-014-8560-x
Jiang X, Trouw LA, van Wesemael TJ, Shi J, Bengtsson C, Kallberg H, Malmstrom V, Israelsson L, Hreggvidsdottir H, Verduijn W, Klareskog L, Alfredsson L, Huizinga TW, Toes RE, Lundberg K, van der Woude D (2014) Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies. Ann Rheum Dis 73(10):1761–1768. https://doi.org/10.1136/annrheumdis-2013-205109
Larsen A (1995) How to apply Larsen score in evaluating radiographs of rheumatoid arthritis in long-term studies. J Rheumatol 22(10):1974–1975
Ajeganova S, van Steenbergen HW, Verheul MK, Forslind K, Hafstrom I, Toes RE, Huizinga TW, Svensson B, Trouw LA, van der Helm-van Mil AH (2017) The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: a study exploring replication and the added value to ACPA and rheumatoid factor. Ann Rheum Dis 76(1):112–118. https://doi.org/10.1136/annrheumdis-2015-208870
Kumar S, Pangtey G, Gupta R, Rehan HS, Gupta LK (2017) Assessment of anti-CarP antibodies, disease activity and quality of life in rheumatoid arthritis patients on conventional and biological disease-modifying antirheumatic drugs. Reumatologia 55(1):4–9. https://doi.org/10.5114/reum.2017.66680
de Moel EC, Derksen V, Trouw LA, Bang H, Collee G, Lard LR, Ramiro S, Huizinga TWJ, Allaart CF, Toes REM, van der Woude D (2019) In rheumatoid arthritis, changes in autoantibody levels reflect intensity of immunosuppression, not subsequent treatment response. Arthritis Res Ther 21(1):28. https://doi.org/10.1186/s13075-019-1815-0
Suzuki T, Ohshima H (2003) Modification by fluoride, bromide, iodide, thiocyanate and nitrite anions of reaction of a myeloperoxidase-H2O2-Cl- system with nucleosides. Chem Pharm Bull 51(3):301–304
Mullaney JC, Medcraft C, Tew DP, Lewis-Borrell L, Golding BT, Walker NR, Legon AC (2017) Cooperative hydrogen bonds form a pseudocycle stabilizing an isolated complex of isocyanic acid with urea. Phys Chem Chem Phys 19(36):25080–25085. https://doi.org/10.1039/c7cp04315e
Taniguchi K, Itagaki S, Yamaguchi K, Mizuno N (2013) Heterogeneous-gold-catalyzed acceptorless cross-dehydrogenative coupling of hydrosilanes and isocyanic acid generated in situ from urea. Angew Chem 52(32):8420–8423. https://doi.org/10.1002/anie.201303132
Zil a R, Rahman MA (2006) Serum thiocyanate levels in smokers, passive smokers and never smokers. JPMA J Pak Med Assoc 56(7):323–326
Vasli LR, Foss OP (1987) Serum thiocyanate, smoking habits and smoking cessation trial in patients with peripheral atherosclerosis. Scand J Clin Lab Invest 47(4):399–403
Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL (2007) Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 13(10):1176–1184. https://doi.org/10.1038/nm1637
Verbrugge FH, Tang WH, Hazen SL (2015) Protein carbamylation and cardiovascular disease. Kidney Int 88(3):474–478. https://doi.org/10.1038/ki.2015.166
Regueiro C, Rodriguez-Rodriguez L, Triguero-Martinez A, Nuno L, Castano-Nunez AL, Villalva A, Perez-Pampin E, Lopez-Golan Y, Abasolo L, Ortiz AM, Herranz E, Pascual-Salcedo D, Martinez-Feito A, Boveda MD, Gomez-Reino JJ, Martin J, Gonzalez-Escribano MF, Fernandez-Gutierrez B, Balsa A, Gonzalez-Alvaro I, Gonzalez A (2019) Specific association of HLA-DRB1*03 with anti-carbamylated protein antibodies in patients with rheumatoid arthritis. Arthritis Rheumatol 71(3):331–339. https://doi.org/10.1002/art.40738
Acknowledgments
The authors acknowledge all the effort exerted by members of the radiology department in their assistance and support in the radiological part of this study.
Author information
Authors and Affiliations
Contributions
This study was conceptualized and designed by SAE, MAI, RMA, and OMM. Patients were selected and diagnosed by SAE, MAI, and RMA. Data sheets were written by RMA. Lab assessments were done by OMM. All authors interpreted the data and discussed the results, and the manuscript was written and revised by SAE.
Corresponding author
Ethics declarations
Informed consents were collected from all patients and ethical permission was obtained from the ethical committee of Sohag University.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elsayed, S.A., Esmail, M.A., Ali, R.M. et al. Diagnostic and prognostic value of anti-CarP antibodies in a sample of Egyptian rheumatoid arthritis patients. Clin Rheumatol 38, 2683–2689 (2019). https://doi.org/10.1007/s10067-019-04616-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04616-z