Abstract
Objective
To evaluate the efficacy and safety of diacerein in patients with rheumatoid arthritis (RA) who are methotrexate inadequate responders (MTX-IR).
Method
In this pilot, multicenter, double-blind, placebo-controlled trial, MTX-IR RA patients were randomized to either diacerein or matching placebo as add-on treatment to MTX for 24 weeks. Efficacy and safety were evaluated every 4 weeks until week 28. Primary and secondary efficacy endpoints were the percentage of patients achieving the ACR20 criteria and a moderate EULAR response at week 24, respectively.
Results
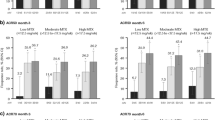
Forty patients were equally randomized to both study treatments; 16 and 19 participants completed the study in the diacerein and the placebo arms, respectively. Baseline characteristics were similar in both groups, except that tender joint count, DAS28-ESR score, and non-steroidal anti-inflammatory drug consumption were higher in the placebo arm. The ACR20 response at week 24 was similar in the diacerein and placebo groups (65% vs 45%, P = .20). However, treatment response according to the EULAR criteria was better in patients taking diacerein (75% vs 25% of moderate response, P = .002). In the 35 patients with assessments through week 28, diacerein was superior to placebo in ACR20 at weeks 24 and 28 (both 81% vs 47%, P = .04). Incidence of adverse events was comparable in both arms, with only chromaturia being more common with diacerein than placebo (40% vs 10%, P = .03).
Conclusions
These preliminary results show the potential benefits of diacerein on pain, joint function, and disease activity in MTX-IR RA patients.
Trial registration
ClinicalTrials.gov Identifier: NCT01264211
Key Points • Diacerein has shown positive effects on rheumatoid arthritis symptoms. • A good safety profile of diacerein has been observed when it was administered as add-on therapy to methotrexate in patients with rheumatoid arthritis. |



Similar content being viewed by others
Data availability
The data generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K (2018) Rheumatoid arthritis. Nat Rev Dis Primers 4:18001. https://doi.org/10.1038/nrdp.2018.1
Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poor G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D (2017) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 76(6):960–977. https://doi.org/10.1136/annrheumdis-2016-210715
Aletaha D, Alasti F, Smolen JS (2016) Optimisation of a treat-to-target approach in rheumatoid arthritis: strategies for the 3-month time point. Ann Rheum Dis 75(8):1479–1485. https://doi.org/10.1136/annrheumdis-2015-208324
Rein P, Mueller RB (2017) Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther 4(2):247–261. https://doi.org/10.1007/s40744-017-0073-3
Fidelix TS, Macedo CR, Maxwell LJ, Fernandes Moca Trevisani V (2014) Diacerein for osteoarthritis. Cochrane Database Syst Rev 2:CD005117
Panova E, Jones G (2015) Benefit-risk assessment of diacerein in the treatment of osteoarthritis. Drug Saf 38(3):245–252. https://doi.org/10.1007/s40264-015-0266-z
Pavelka K, Bruyere O, Cooper C, Kanis JA, Leeb BF, Maheu E, Martel-Pelletier J, Monfort J, Pelletier JP, Rizzoli R, Reginster JY (2016) Diacerein: Benefits, risks and place in the management of osteoarthritis. An opinion-based report from the ESCEO. Drugs Aging 33(2):75–85. Erratum in: Drugs Aging. 2017;2034:2413. https://doi.org/10.1007/s40266-016-0347-4
Pelletier JP, Mineau F, Fernandes JC, Duval N, Martel-Pelletier J (1998) Diacerhein and rhein reduce the interleukin 1 beta stimulated inducible nitric oxide synthesis level and activity while stimulating cyclooxygenase-2 synthesis in human osteoarthritic chondrocytes. J Rheumatol 25(12):2417–2424
Moore AR, Greenslade KJ, Alam CA, Willoughby DA (1998) Effects of diacerhein on granuloma induced cartilage breakdown in the mouse. Osteoarthr Cartil 6(1):19–23
Álvarez-Soria MA, Herrero-Beaumont G, Sánchez-Pernaute O, Bellido M, Largo R (2008) Diacerein has a weak effect on the catabolic pathway of human osteoarthritis synovial fibroblast - comparison to its effects on osteoarthritic chondrocytes. Rheumatology (Oxford) 47(5):627–633
Lee DM, Weinblatt ME (2001) Rheumatoid arthritis. Lancet 358(9285):903–911
Tamura T, Ohmori K, Nakamura K (1999) Effect of diacerein on spontaneous polyarthritis in male New Zealand black/KN mice. Osteoarthr Cartil 7(6):533–538
Douni E, Sfikakis PP, Haralambous S, Fernandes P, Kollias G (2004) Attenuation of inflammatory polyarthritis in TNF transgenic mice by diacerein: comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis Res Ther 6:R65–R72
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American Rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F (1992) The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 35(5):498–502
Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1):44–48
Fransen J, van Riel PL (2005) The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 23(Suppl 39):S93–S99
Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R Jr, Paulus H, Strand V et al (1995) American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 38(6):727–735
Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, Felson DT, Hajjaj-Hassouni N, Hochberg M, Logeart I, Matucci-Cerinic M, van de Laar M, van der Heijde D, Dougados M (2012) Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res 64(11):1699–1707. https://doi.org/10.1002/acr.21747
ICH harmonised tripartite guideline. Clinical safety data management: definitions and standards for expedited reporting E2A, (1994)
Louthrenoo W, Nilganuwong S, Aksaranugraha S, Asavatanabodee P, Saengnipanthkul S, The Thai Study Group (2007) The efficacy, safety and carry-over effect of diacerein in the treatment of painful knee osteoarthritis: a randomised, double-blind, NSAID-controlled study. Osteoarthr Cartil 15(6):605–614
Zheng WJ, Tang FL, Li J, Zhang FC, Li ZG, Su Y, Wu DH, Ma L, Zhou HQ, Huang F, Zhang JL, Liang DF, Zhou YX, Xu H (2006) Evaluation of efficacy and safety of diacerein in knee osteoarthritis in Chinese patients. Chin Med Sci J 21(2):75–80
Lequesne M, Berdah L, Gérentes I (1998) [Efficacy and tolerance of diacerhein in the treatment of gonarthrosis and coxarthrosis] Efficacité et tolérance de la diacerhéine dans le traitement de la gonarthrose et de la coxarthrose. Rev Prat 48(Suppl 17):S31–S35
Nguyen M, Dougados M, Berdah L, Amor B (1994) Diacerhein in the treatment of osteoarthritis of the hip. Arthritis Rheum 37(4):529–536
Committee for Proprietary Medicinal Products (CPMP) (2003) Points to consider on clinical investigation of medicinal products other than NSAIDs for treatment of rheumatoid arthritis. European Agency for the Evaluation of Medicinal Products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003439.pdf. Accessed Jan 10 2016
Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Alvaro-Gracia JM, Bakkers M, Brodin N, Burmester GR, Codreanu C, Conway R, Dougados M, Emery P, Ferraccioli G, Fonseca J, Raza K, Silva-Fernandez L, Smolen JS, Skingle D, Szekanecz Z, Kvien TK, van der Helm-van Mil A, van Vollenhoven R (2017) 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 76(6):948–959. https://doi.org/10.1136/annrheumdis-2016-210602
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O’Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, Mcalindon T, American College of R (2016) 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 68(1):1–25. https://doi.org/10.1002/acr.22783
Katchamart W, Narongroeknawin P, Chevaisrakul P, Dechanuwong P, Mahakkanukrauh A, Kasitanon N, Pakchotanon R, Sumethkul K, Ueareewongsa P, Ukritchon S, Bhurihirun T, Duangkum K, Intapiboon P, Intongkam S, Jangsombatsiri W, Jatuworapruk K, Kositpesat N, Leungroongroj P, Lomarat W, Petcharat C, Sittivutworapant S, Suebmee P, Tantayakom P, Tipsing W, Asavatanabodee P, Chiowchanwisawakit P, Foocharoen C, Koolvisoot A, Louthrenoo W, Siripaitoon B, Totemchokchyakarn K, Kitumnuaypong T, Thai Rheumatism A (2017) Evidence-based recommendations for the diagnosis and management of rheumatoid arthritis for non-rheumatologists: integrating systematic literature research and expert opinion of the Thai Rheumatism association. Int J Rheum Dis 20(9):1142–1165. https://doi.org/10.1111/1756-185X.12905
Furst DE, Kremer JM (1988) Methotrexate in rheumatoid arthritis. Arthritis Rheum 31(3):305–314
Acknowledgements
We thank Nuntana Kasitanon, Praveena Chiowchanwisawakit, Ajanee Mahakkanukrauh, and Parichat Ueaareewongsa who were co-investigators. Statistical analysis was performed by Statmed SARL, France. Medical writing assistance was provided by Totzke & Dreher Scientific SA, Switzerland.
Funding support
This study was supported by TRB Chemedica (Thailand) Ltd. However, the company had no influence on the design and the conduct of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Worawit Louthrenoo and Boonjing Siripaitoon received speaking honoraria from TRB Chemedica (Thailand) Ltd. Sabine Collaud Basset is an employee of TRB Chemedica International SA, Switzerland. Surasak Nilganuwong and Ratanavadee Nanagara declared no conflicts of interest.
Patient consent
All patients gave their informed consent prior to enter the clinical trial, i.e., before they underwent any study procedure at the screening visit.
Ethics approval
The trial was conducted in accordance with the Good Clinical Practices and was compliant with the principles of the 1983 Declaration of Helsinki. Ethics approval was provided by the Institutional Review Boards of the four participating centers. The study has been registered in the ClinicalTrials.gov National Institute of Health trial register (NCT01264211).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Louthrenoo, W., Nilganuwong, S., Nanagara, R. et al. Diacerein for the treatment of rheumatoid arthritis in patients with inadequate response to methotrexate: a pilot randomized, double-blind, placebo-controlled add-on trial. Clin Rheumatol 38, 2461–2471 (2019). https://doi.org/10.1007/s10067-019-04587-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04587-1