Abstract
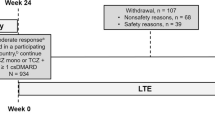
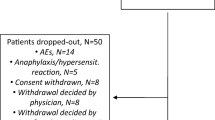
To analyse efficacy and safety of tocilizumab in patients with rheumatoid arthritis (RA) and an inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) and/or tumour necrosis factor (TNF) inhibitors of the Swiss and Austrian patients from the ACT-SURE study. This is a sub-analysis of RA patients from Switzerland and Austria, who participated in the international phase 3b, open-label, ACT-SURE study. Patients with an inadequate response to csDMARDs or TNF antagonists receiving 8 mg/kg of IV tocilizumab every 4 weeks during a 24-week time period were included into the study. Therapy with one or more csDMARDs could be continued as combination therapy with tocilizumab (Combo) or stopped, resulting in tocilizumab monotherapy (Mono), at the treating physician’s discretion. These two patient groups were analysed in separate and compared. Overall, 107 (22 on Mono vs. 85 on Combo) patients were treated with tocilizumab. The percentage of patients with at least one adverse event was significantly lower in the tocilizumab combination (58.8%) as compared to the monotherapy group (81.8%, p = 0.0458). No differences in ACR20/50/70/90 response rates were observed between both treatment groups at week 24 (Mono 63.6, 40.9, 22.7, and 18.2% vs. Combo 61.2, 43.5, 25.9, and 10.6%). The median time to low disease activity (LDA) was significantly shorter in patients treated with tocilizumab combination therapy (Mono 9.1, Combo 7.9 weeks, log rank p = 0.038). In this post hoc regional sub-analysis of the ACT-SURE study, no differences for disease activity were found comparing the two patient groups at week 24. However, median time to LDA was statistically shorter in patients treated with tocilizumab combination therapy as compared to tocilizumab monotherapy. Consequently, adding tocilizumab to csDMARD therapy rather than changing to tocilizumab monotherapy may be, in our opinion, the safest strategy to reach maximum effect in RA patients with active disease despite treatment with csDMARD. csDMARDs can be withdrawn either immediately due to adverse events or after at least low disease activity has been reached.

Similar content being viewed by others
References
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC et al (2016) 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68(1):1–26
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73(3):492–509
Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A et al (2008) IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 67(11):1516–1523
Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E et al (2008) Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 58(10):2968–2980
Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ et al (2010) Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 69(1):88–96
Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G et al (2006) Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 54(9):2817–2829
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E et al (2008) Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371(9617):987–997
Bykerk VP, Ostor AJ, Alvaro-Gracia J, Pavelka K, Ivorra JA, Graninger W et al (2012) Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann Rheum Dis 71(12):1950–1954
Bykerk VP, Ostor AJ, Alvaro-Gracia J, Pavelka K, Roman Ivorra JA, Graninger W et al (2015) Comparison of tocilizumab as monotherapy or with add-on disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and inadequate responses to previous treatments: an open-label study close to clinical practice. Clin Rheumatol 34(3):563–571
Alvaro-Gracia JM, Fernandez-Nebro A, Garcia-Lopez A, Guzman M, Blanco FJ, Navarro FJ et al (2014) Tocilizumab in patients with active rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs or tumor necrosis factor inhibitors: subanalysis of Spanish results of an open-label study close to clinical practice. Reumatol Clin 10(2):94–100
Marschner IC (2010) Regional differences in multinational clinical trials: anticipating chance variation. Clin Trials 7(2):147–156
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1):44–48
Witte T (2015) Methotrexate as combination partner of TNF inhibitors and tocilizumab. What is reasonable from an immunological viewpoint? Clin Rheumatol 34(4):629–634
Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V et al (2011) High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis 70(5):785–791
Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L et al (2011) Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology (Oxford) 50(1):101–109
Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R et al (2004) Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364(9430):263–269
Acknowledgements
This work was supported by F. Hoffmann-La Roche.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted in accordance with good clinical practice and the Declaration of Helsinki. An institutional review committee at each participating centre approved the study.
Disclosures
Paris Sidiropoulos is an employee of F. Hoffman-La Roche Ltd. and Christoph Goger is an employee of Roche Austria GmbH. Ruediger Mueller, Winfried Graninger, and Johannes von Kempis have nothing to disclose.
Rights and permissions
About this article
Cite this article
Mueller, R.B., Graninger, W., Sidiropoulos, P. et al. Median time to low disease activity is shorter in tocilizumab combination therapy with csDMARDs as compared to tocilizumab monotherapy in patients with active rheumatoid arthritis and inadequate responses to csDMARDs and/or TNF inhibitors: sub-analysis of the Swiss and Austrian patients from the ACT-SURE study. Clin Rheumatol 36, 2187–2192 (2017). https://doi.org/10.1007/s10067-017-3779-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3779-2