Abstract
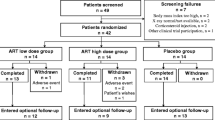
Low-dose SoluMatrix diclofenac was developed to provide effective pain relief for osteoarthritis pain. We evaluated the effects of SoluMatrix diclofenac on health-related quality of life (HRQoL) measures in patients with osteoarthritis, hypothesizing that SoluMatrix-treated patients would experience significant improvement compared with placebo. In this 12-week, phase 3 randomized controlled trial, 305 patients with osteoarthritis of the hip or knee received SoluMatrix diclofenac 35 mg three times (TID) or twice (BID) daily or placebo. Measures included HRQoL, assessed by Short Form 36 (SF-36, version 2), and pain, stiffness, and physical function, assessed by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at baseline and at week 12. Descriptive statistics were calculated for mean changes from baseline; inferential statistics compared treatment groups with placebo. SoluMatrix diclofenac 35 mg BID (6.2 [0.75]; P = 0.0048) or TID (6.6 [0.80]; P = 0.0014) produced large improvements in the SF-36 physical component summary (PCS) scores at week 12 (least squares mean change from baseline [SE]) compared with placebo (3.5 [0.78]). Minimum clinically important differences were observed in six out of eight SF-36 domains among patients in SoluMatrix diclofenac groups and five out of eight domains in the placebo group; treatment with SoluMatrix diclofenac 35 mg TID produced significant improvements (P ≤ 0.03) in five out of eight domains versus placebo. SoluMatrix diclofenac 35 mg TID significantly improved responses to 23 out of 24 questions in the WOMAC versus placebo (P ≤ 0.0334). Low-dose SoluMatrix diclofenac 35 mg TID and BID significantly improved HRQoL, pain, stiffness, and physical function in patients with osteoarthritis of the hip or knee.


Similar content being viewed by others
Notes
SoluMatrix® is a registered trademark of iCeutica Pty Ltd. and is licensed to Iroko.
ZORVOLEX® is a registered trademark of Iroko Properties, Inc.
References
Symmons D, Mathers C, Pfleger B (2000) Global burden of osteoarthritis in the year 2000. WHO, Geneva. http://www.who.int/healthinfo/statistics/bod_osteoarthritis.pdf. Accessed 25 Feb 2014
Dominick KL, Ahern FM, Gold CH, Heller DA (2004) Health-related quality of life among older adults with arthritis. Health Qual Life Outcomes 2:5
Farr Ii J, Miller LE, Block JE (2013) Quality of life in patients with knee osteoarthritis: a commentary on nonsurgical and surgical treatments. Open Orthop J 7:619–623
Kosinski M, Kujawski SC, Martin R, Wanke LA, Buatti MC, Ware JE Jr, Perfetto EM (2002) Health-related quality of life in early rheumatoid arthritis: impact of disease and treatment response. Am J Manag Care 8(3):231–240
Balmaceda CM (2014) Clinical trial data in support of changing guidelines in osteoarthritis treatment. J Pain Res 7:211–218
Yu SP, Hunter DJ (2015) Managing osteoarthritis. Aust Prescr 38(4):115–119
Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F, Sturkenboom M, Perez-Gutthann S, Safety of Non-Steroidal Anti-Inflammatory Drugs (SOS) Project (2012) Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf 35(12):1127–1146
Coxib and traditional NSAID Trialists’ (CNT) Collaboration, Bhala N, Emberson J et al (2013) Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382(9894):769–779
McGettigan P, Henry D (2011) Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 8(9):e1001098
Food and Drug Administration (2005) Public health advisory—FDA announces important changes and additional warnings for COX-2 selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm150314.htm. Accessed 27 May 2016
Food and Drug Administration (2015) FDA drug safety communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. http://www.fda.gov/Drugs/DrugSafety/ucm451800.htm. Accessed 27 May 2016
Desjardins PJ, Olugemo K, Solorio D, Young CL (2015) Pharmacokinetic properties and tolerability of low-dose SoluMatrix diclofenac. Clin Ther 37(2):448–461
Gibofsky A, Hochberg MC, Jaros M, Young CL (2014) Efficacy and safety of low-dose submicron diclofenac for the treatment of osteoarthritis pain: a 12-week, phase 3 study. Curr Med Res Opin 30(9):1883–1893
Altman RD, Strand V, Hochberg MC, Gibofsky A, Markenson JA, Hopkins WE, Cryer B, Kivitz A, Nezzer J, Imasogie O, Young CL (2015) Low-dose SoluMatrix diclofenac in the treatment of osteoarthritis: a 1-year, open-label, phase III safety study. Postgrad Med 127(5):517–528
(2016) Full prescribing information for ZORVOLEX. Iroko Pharmaceuticals, LLC, Philadelpha
van der Waal JM, Terwee CB, van der Windt DA, Bouter LM, Dekker J (2005) The impact of non-traumatic hip and knee disorders on health-related quality of life as measured with the SF-36 or SF-12. A systematic review. Qual Life Res 14(4):1141–1155
Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L (1992) Validating the SF-36 Health Survey Questionnaire: new outcome measure for primary care. BMJ 305(6846):160–164
Stubbs B, Hurley M, Smith T (2015) What are the factors that influence physical activity participation in adults with knee and hip osteoarthritis? A systematic review of physical activity correlates. Clin Rehabil 29(1):80–94
Hawker GA, Mian S, Kendzerska T, French M (2011) Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), chronic pain grade scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 63(Suppl 11):S240–S252
Kellgren J, Lawrence J (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494
(2000) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 284(23):3043–3045
Strand V, Boers M, Idzerda L, Kirwan JR, Kvien TK, Tugwell PS, Dougados M (2011) It’s good to feel better but it’s better to feel good and even better to feel good as soon as possible for as long as possible. Response criteria and the importance of change at OMERACT 10. J Rheumatol 38(8):1720–1727
Strand V, Kelman A (2004) Outcome measures in osteoarthritis: randomized controlled trials. Curr Rheumatol Rep 6(1):20–30
Strand V, Crawford B, Singh J, Choy E, Smolen JS, Khanna D (2009) Use of “spydergrams” to present and interpret SF-36 health-related quality of life data across rheumatic diseases. Ann Rheum Dis 68(12):1800–1804
Ware JE, Kosinski M, Dewey JE (2002) How to score version 2 of the SF-36 Health Survey (standard & acute forms), Third edition edn. Quality Metric Incorporated, Lincoln, RI
Ara R, Brazier J (2008) Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 11(7):1131–1143
Ara R, Brazier J (2009) Predicting the short form-6D preference-based index using the eight mean short form-36 health dimension scores: estimating preference-based health-related utilities when patient level data are not available. Value Health 12(2):346–353
Farivar SS, Cunningham WE, Hays RD (2007) Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.I. Health Qual Life Outcomes 5:54
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15(12):1833–1840
Kim TH, Kim KH, Kang JW, Lee M, Kang KW, Kim JE, Kim JH, Lee S, Shin MS, Jung SY, Kim AR, Park HJ, Jung HJ, Song HS, Kim HJ, Choi JB, Hong KE, Choi SM (2014) Moxibustion treatment for knee osteoarthritis: a multi-centre, non-blinded, randomised controlled trial on the effectiveness and safety of the moxibustion treatment versus usual care in knee osteoarthritis patients. PLoS One 9(7):e101973
Theiler R, Bischoff HA, Good M, Uebelhart D (2002) Rofecoxib improves quality of life in patients with hip or knee osteoarthritis. Swiss Med Wkly 132(39–40):566–573
Hill CL, Parsons J, Taylor A, Leach G (1999) Health related quality of life in a population sample with arthritis. J Rheumatol 26(9):2029–2035
Rabenda V, Burlet N, Ethgen O, Raeman F, Belaiche J, Reginster JY (2005) A naturalistic study of the determinants of health related quality of life improvement in osteoarthritic patients treated with non-specific non-steroidal anti-inflammatory drugs. Ann Rheum Dis 64(5):688–693
Hopman-Rock M, Kraaimaat FW, Bijlsma JW (1997) Quality of life in elderly subjects with pain in the hip or knee. Qual Life Res 6(1):67–76
Acknowledgements
The authors would like to thank the following individuals: Mark Jaros, PhD; Jennifer Nezzer, MS; Susan Whitcher; Aaron Danna; Michael Kuss; Garen Manvelian, MD; Daniel Solorio; Olaolu Imasogie, MD; Claire Sheridan, PhD; Melanie Lauterio, PhD; and the investigators and patients who participated in this study. Ewa Wandzioch, PhD; Jill See, PhD; and Colville Brown, MD, of AlphaBioCom, LLC (King of Prussia, PA), provided the editorial support for this manuscript. Funding was provided by Iroko.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients provided written informed consent prior to any protocol-specified procedures, and the study was approved by the Copernicus Group Institutional Review Board (Research Triangle Park, NC, USA) and was conducted in accordance with the standards and ethical principles of the International Conference on Harmonization Good Clinical Practice Guidelines and the Declaration of Helsinki at 40 sites in the USA.
Conflict of interest
Vibeke Strand is a consultant to Afferent; Ampio; Bioventus; Carbylan; Eupraxia; Flexion; Iroko Pharmaceuticals, LLC; Lilly; Pfizer; Sanofi; and SKK. She is a founding member of the executive committee of OMERACT, an organization that develops and validates outcome measures in rheumatology. Martin Bergman serves as a consultant to Iroko Pharmaceuticals, LLC; Amgen; Celgene; Roche; AstraZeneca; AbbVie; and Pfizer and is a stockholder in Merck & Co, Inc.; Johnson & Johnson; Pfizer; and Bristol-Myers Squibb. Jasvinder A. Singh has received research grants from Takeda and Savient and consultant fees from Savient; Takeda; Regeneron; Merz; Bioiberica; Crealta; WebMD; UBM, LLC; Allergan; and Iroko Pharmaceuticals, LLC. Dr. Singh serves as the principal investigator for an investigator-initiated study funded by Horizon Pharmaceuticals through a grant to DINORA, Inc., and is also a member of the executive committee of OMERACT, an organization that develops and validates outcome measures in rheumatology. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. Allan Gibofsky is a stock shareholder of GlaxoSmithKline PLC, Bristol-Myers Squibb, Johnson & Johnson, Amgen, Pfizer, and AbbVie and serves as a consultant for Takeda, Amgen, AbbVie, UCB, Genentech, Horizon, and Iroko Pharmaceuticals, LLC. Alan Kivitz is a speaker for Iroko Pharmaceuticals, LLC. Clarence Young was an employee of Iroko Pharmaceuticals, LLC, at the time this study was conducted.
Additional information
Clarence Young was an employee of Iroko Pharmaceuticals, LLC, at the time this study was conducted.
Electronic supplementary material
Online resource 1
(PDF 52 kb)
Rights and permissions
About this article
Cite this article
Strand, V., Bergman, M., Singh, J.A. et al. Low-dose SoluMatrix diclofenac in patients with osteoarthritis pain: impact on quality of life in a controlled trial. Clin Rheumatol 36, 1357–1367 (2017). https://doi.org/10.1007/s10067-017-3569-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3569-x