Abstract
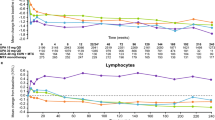
The change in transaminase levels over a single week during therapy with methotrexate (MTX) has not been investigated or reported to date. In clinical practice, it is common to observe abnormal transaminase levels upon routine blood work for toxicity monitoring. Many have suggested that such lab abnormalities can sometimes be attributed to sampling blood for toxicity monitoring proximately following MTX dosing. The aim of our study was to evaluate changes in transaminase levels (AST/ALT) over 1 week after MTX administration in rheumatoid arthritis (RA) patients. In this small proof of concept study, we evaluated 13 patients with RA taking stable doses of methotrexate and background medications (e.g., NSAIDs and prednisone), but no other disease-modifying anti-rheumatic drugs (DMARDs). All patients were on a stable dose of folic acid. Patients received their usual doses of MTX administered at a specified time, and then sequential blood samples were obtained over the course of 7 days. Peripheral blood was obtained at each time point to measure serum transaminases. We did not observe any significant change in sequential transaminases over 1 week in relationship to MTX administration. It is possible that MTX therapy alone does not lead to significant weekly transaminase variations, contrary to our clinical expectations. The addition of other medications (i.e., NSAIDs) to stable MTX regimen may result in transaminase abnormalities.

Similar content being viewed by others
References
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC et al (2016) 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 68(1):1–26
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73(3):492–509
Kremer JM, Lee JK (1988) A long-term prospective study of the use of methotrexate in rheumatoid arthritis. Update after a mean of fifty-three months. Arthritis Rheum 31(5):577–584
Weinblatt ME, Trentham DE, Fraser PA, Holdsworth DE, Falchuk KR, Weissman BN et al (1988) Long-term prospective trial of low-dose methotrexate in rheumatoid arthritis. Arthritis Rheum 31(2):167–175
Kremer JM, Alarcon GS, Lightfoot RW Jr, Willkens RF, Furst DE, Williams HJ et al (1994) Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum 37(3):316–328
Salliot C, van der Heijde D (2009) Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 68(7):1100–1104
Kent PD, Luthra HS, Michet C Jr (2004) Risk factors for methotrexate-induced abnormal laboratory monitoring results in patients with rheumatoid arthritis. J Rheumatol 31(9):1727–1731
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT (2010) Bingham CO,3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581
Visser K, van der Heijde D (2009) Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis 68(7):1094–1099
Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J et al (2009) Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis 68(7):1086–1093
Tracy TS, Krohn K, Jones DR, Bradley JD, Hall SD, Brater DC (1992) The effects of a salicylate, ibuprofen, and naproxen on the disposition of methotrexate in patients with rheumatoid arthritis. Eur J Clin Pharmacol 42(2):121–125
Stewart CF, Fleming RA, Arkin CR, Evans WE (1990) Coadministration of naproxen and low-dose methotrexate in patients with rheumatoid arthritis. Clin Pharmacol Ther 47(4):540–546
Acknowledgements
The authors acknowledge the research staff of the Johns Hopkins Arthritis Center: Michelle Jones, Grazyna Purwin, Marilyn Towns, Brandy Miles, and Victoria Ruffing RN for their assistance in conducting this study, Drs. Grant Louie, Thomas Grader-Beck and Uzma Haque for contributing patients, and the patients who participated.
Author contributions
All authors were involved in designing the study, analyzing the data, drafting the article, or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Bingham had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Johns Hopkins Institutional Review Board, and all patients provided written informed consent.
Conflicts of interest
The authors declare they have no conflicts of interest. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors agree to allow the journal to review their data if requested.
Consent for publication
Not Applicable.
Funding
This work was supported by The Johns Hopkins Arthritis Center Discovery Fund. This study was also supported in part by Grant Number P30-AR053503 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Research reported in this publication was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number T32AR048522. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAMS or the National Institutes of Health.
Rights and permissions
About this article
Cite this article
Mecoli, C.A., Delev, N.G. & Bingham, C.O. Measuring transaminases in patients with rheumatoid arthritis on weekly methotrexate: does timing of blood testing matter?. Clin Rheumatol 35, 3053–3056 (2016). https://doi.org/10.1007/s10067-016-3361-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3361-3